Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial
- PMID: 22447964
- PMCID: PMC3400995
- DOI: 10.1164/rccm.201108-1553OC
Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial
Abstract
Rationale: Exacerbations of chronic obstructive pulmonary disease (COPD) and responses to treatment are heterogeneous.
Objectives: Investigate the usefulness of blood eosinophils to direct corticosteroid therapy during exacerbations.
Methods: Subjects with COPD exacerbations were entered into a randomized biomarker-directed double-blind corticosteroid versus standard therapy study. Subjects in the standard arm received prednisolone for 2 weeks, whereas in the biomarker-directed arm, prednisolone or matching placebo was given according to the blood eosinophil count biomarker. Both study groups received antibiotics. Blood eosinophils were measured in the biomarker-directed and standard therapy arms to define biomarker-positive and -negative exacerbations (blood eosinophil count > and ≤ 2%, respectively). The primary outcome was to determine noninferiority in health status using the chronic respiratory questionnaire (CRQ) and in the proportion of exacerbations associated with a treatment failure between subjects allocated to the biomarker-directed and standard therapy arms.
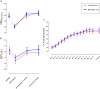
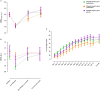
Measurements and main results: There were 86 and 80 exacerbations in the biomarker-directed and standard treatment groups, respectively. In the biomarker-directed group, 49% of the exacerbations were not treated with prednisolone. CRQ improvement after treatment in the standard and biomarker-directed therapy groups was similar (0.8 vs. 1.1; mean difference, 0.3; 95% confidence interval, 0.0-0.6; P = 0.05). There was a greater improvement in CRQ in biomarker-negative exacerbations given placebo compared with those given prednisolone (mean difference, 0.45; 95% confidence interval, 0.01-0.90; P = 0.04). In biomarker-negative exacerbations, treatment failures occurred in 15% given prednisolone and 2% of those given placebo (P = 0.04).
Conclusions: The peripheral blood eosinophil count is a promising biomarker to direct corticosteroid therapy during COPD exacerbations, but larger studies are required.
Figures
Similar articles
-
Blood eosinophil-guided oral prednisolone for COPD exacerbations in primary care in the UK (STARR2): a non-inferiority, multicentre, double-blind, placebo-controlled, randomised controlled trial.Lancet Respir Med. 2024 Jan;12(1):67-77. doi: 10.1016/S2213-2600(23)00298-9. Epub 2023 Nov 2. Lancet Respir Med. 2024. PMID: 37924830 Clinical Trial.
-
Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial.Lancet Respir Med. 2019 Aug;7(8):699-709. doi: 10.1016/S2213-2600(19)30176-6. Epub 2019 May 20. Lancet Respir Med. 2019. PMID: 31122894 Clinical Trial.
-
A multi-center randomized, controlled, open-label trial evaluating the effects of eosinophil-guided corticosteroid-sparing therapy in hospitalised patients with COPD exacerbations - The CORTICO steroid reduction in COPD (CORTICO-COP) study protocol.BMC Pulm Med. 2017 Aug 15;17(1):114. doi: 10.1186/s12890-017-0458-7. BMC Pulm Med. 2017. PMID: 28810909 Free PMC article. Clinical Trial.
-
Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Mar 19;3(3):CD006897. doi: 10.1002/14651858.CD006897.pub4. Cochrane Database Syst Rev. 2018. PMID: 29553157 Free PMC article. Review.
-
Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2014 Dec 10;(12):CD006897. doi: 10.1002/14651858.CD006897.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Mar 19;3:CD006897. doi: 10.1002/14651858.CD006897.pub4 PMID: 25491891 Updated. Review.
Cited by
-
Blood Eosinophilia and Its Stability in Hospitalized COPD Exacerbations are Associated with Lower Risk of All-Cause Mortality.Int J Chron Obstruct Pulmon Dis. 2020 May 19;15:1123-1134. doi: 10.2147/COPD.S245056. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32547000 Free PMC article.
-
Incidence and profile of severe exacerbations of chronic obstructive pulmonary disease due to biomass smoke or tobacco.Ann Thorac Med. 2022 Oct-Dec;17(4):193-198. doi: 10.4103/atm.atm_155_22. Epub 2022 Oct 7. Ann Thorac Med. 2022. PMID: 36387759 Free PMC article.
-
Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission.Lancet. 2022 Sep 17;400(10356):921-972. doi: 10.1016/S0140-6736(22)01273-9. Epub 2022 Sep 5. Lancet. 2022. PMID: 36075255 Free PMC article. Review.
-
Blood Eosinophils and Exhaled Nitric Oxide: Surrogate Biomarkers of Airway Eosinophilia in Stable COPD and Exacerbation.Biomedicines. 2022 Aug 30;10(9):2128. doi: 10.3390/biomedicines10092128. Biomedicines. 2022. PMID: 36140229 Free PMC article. Review.
-
Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary.Eur Respir J. 2023 Apr 1;61(4):2300239. doi: 10.1183/13993003.00239-2023. Print 2023 Apr. Eur Respir J. 2023. PMID: 36858443 Free PMC article.
References
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 - PubMed
-
- Saetta M, Di SA, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp CE, Ciaccia A, Fabbri LM. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 1994;150:1646–1652 - PubMed
-
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–1121 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

