NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy
- PMID: 22447076
- PMCID: PMC3348859
- DOI: 10.1038/nm.2650
NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy
Abstract
It is unclear whether the ability of the innate immune system to recognize distinct ligands from a single microbial pathogen via multiple pattern recognition receptors (PRRs) triggers common pathways or differentially triggers specific host responses. In the human mycobacterial infection leprosy, we found that activation of monocytes via nucleotide-binding oligomerization domain-containing protein 2 (NOD2) by its ligand muramyl dipeptide, as compared to activation via heterodimeric Toll-like receptor 2 and Toll-like receptor 1 (TLR2/1) by triacylated lipopeptide, preferentially induced differentiation into dendritic cells (DCs), which was dependent on a previously unknown interleukin-32 (IL-32)-dependent mechanism. Notably, IL-32 was sufficient to induce monocytes to rapidly differentiate into DCs, which were more efficient than granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived DCs in presenting antigen to major histocompatibility complex (MHC) class I-restricted CD8(+) T cells. Expression of NOD2 and IL-32 and the frequency of CD1b(+) DCs at the site of leprosy infection correlated with the clinical presentation; they were greater in patients with limited as compared to progressive disease. The addition of recombinant IL-32 restored NOD2-induced DC differentiation in patients with the progressive form of leprosy. In conclusion, the NOD2 ligand-induced, IL-32-dependent DC differentiation pathway contributes a key and specific mechanism for host defense against microbial infection in humans.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


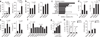
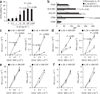

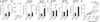
Similar articles
-
Toll-like receptor-induced granulocyte-macrophage colony-stimulating factor secretion is impaired in Crohn's disease by nucleotide oligomerization domain 2-dependent and -independent pathways.Clin Exp Immunol. 2009 Mar;155(3):487-95. doi: 10.1111/j.1365-2249.2008.03850.x. Epub 2008 Dec 15. Clin Exp Immunol. 2009. PMID: 19094116 Free PMC article.
-
Human NOD2 Recognizes Structurally Unique Muramyl Dipeptides from Mycobacterium leprae.Infect Immun. 2016 Aug 19;84(9):2429-38. doi: 10.1128/IAI.00334-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27297389 Free PMC article.
-
Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes.Eur J Immunol. 2008 Mar;38(3):750-62. doi: 10.1002/eji.200737395. Eur J Immunol. 2008. PMID: 18236400
-
NOD2 stimulation by Staphylococcus aureus-derived peptidoglycan is boosted by Toll-like receptor 2 costimulation with lipoproteins in dendritic cells.Infect Immun. 2014 Nov;82(11):4681-8. doi: 10.1128/IAI.02043-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156723 Free PMC article.
-
Cytokines in the generation and maturation of dendritic cells: recent advances.Eur Cytokine Netw. 2002 Apr-Jun;13(2):186-99. Eur Cytokine Netw. 2002. PMID: 12101074 Review.
Cited by
-
Genome-wide meta-analysis and fine-mapping prioritize potential causal variants and genes related to leprosy.MedComm (2020). 2023 Nov 24;4(6):e415. doi: 10.1002/mco2.415. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020709 Free PMC article.
-
LRRK2 and RIPK2 variants in the NOD 2-mediated signaling pathway are associated with susceptibility to Mycobacterium leprae in Indian populations.PLoS One. 2013 Aug 28;8(8):e73103. doi: 10.1371/journal.pone.0073103. eCollection 2013. PLoS One. 2013. PMID: 24015287 Free PMC article. Clinical Trial.
-
Novel insights into the biology of interleukin-32.Cell Mol Life Sci. 2013 Oct;70(20):3883-92. doi: 10.1007/s00018-013-1301-9. Epub 2013 Mar 6. Cell Mol Life Sci. 2013. PMID: 23463238 Free PMC article. Review.
-
Natural Killer Cell Transcript 4 promotes the development of Sjӧgren's syndrome via activation of Rap1 on B cells.J Autoimmun. 2021 Jan;116:102559. doi: 10.1016/j.jaut.2020.102559. Epub 2020 Oct 19. J Autoimmun. 2021. PMID: 33087256 Free PMC article.
-
CD1 and mycobacterial lipids activate human T cells.Immunol Rev. 2015 Mar;264(1):138-53. doi: 10.1111/imr.12253. Immunol Rev. 2015. PMID: 25703557 Free PMC article. Review.
References
-
- Medzhitov R, Preston-Hurlburt P, Janeway CAJ. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Brightbill HD, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. - PubMed
-
- Bloom BR. Learning from leprosy: A perspective on immunology and the third world. J. Immunol. 1986;137:i–x. - PubMed
-
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966;34:255–273. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R01 AI022553-27/AI/NIAID NIH HHS/United States
- R01 AI047868/AI/NIAID NIH HHS/United States
- R01 AI082575/AI/NIAID NIH HHS/United States
- AI047868/AI/NIAID NIH HHS/United States
- R01 AR040312-22/AR/NIAMS NIH HHS/United States
- AR040312/AR/NIAMS NIH HHS/United States
- R01 AI022553/AI/NIAID NIH HHS/United States
- R01S AI022553/AI/NIAID NIH HHS/United States
- R01 AR059126/AR/NIAMS NIH HHS/United States
- R37 AI047868/AI/NIAID NIH HHS/United States
- R01 AI056154/AI/NIAID NIH HHS/United States
- R01 AR040312/AR/NIAMS NIH HHS/United States
- R01 AI047868-12/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

