Cell shape dynamics: from waves to migration
- PMID: 22438794
- PMCID: PMC3305346
- DOI: 10.1371/journal.pcbi.1002392
Cell shape dynamics: from waves to migration
Abstract
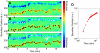
We observe and quantify wave-like characteristics of amoeboid migration. Using the amoeba Dictyostelium discoideum, a model system for the study of chemotaxis, we demonstrate that cell shape changes in a wave-like manner. Cells have regions of high boundary curvature that propagate from the leading edge toward the back, usually along alternating sides of the cell. Curvature waves are easily seen in cells that do not adhere to a surface, such as cells that are electrostatically repelled from surfaces or cells that extend over the edge of micro-fabricated cliffs. Without surface contact, curvature waves travel from the leading edge to the back of a cell at -35 µm/min. Non-adherent myosin II null cells do not exhibit these curvature waves. At the leading edge of adherent cells, curvature waves are associated with protrusive activity. Like regions of high curvature, protrusive activity travels along the boundary in a wave-like manner. Upon contact with a surface, the protrusions stop moving relative to the surface, and the boundary shape thus reflects the history of protrusive motion. The wave-like character of protrusions provides a plausible mechanism for the zig-zagging of pseudopods and for the ability of cells both to swim in viscous fluids and to navigate complex three dimensional topography.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
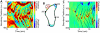
Similar articles
-
Microtopographical guidance of macropinocytic signaling patches.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2110281118. doi: 10.1073/pnas.2110281118. Proc Natl Acad Sci U S A. 2021. PMID: 34876521 Free PMC article.
-
How blebs and pseudopods cooperate during chemotaxis.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11703-8. doi: 10.1073/pnas.1322291111. Epub 2014 Jul 29. Proc Natl Acad Sci U S A. 2014. PMID: 25074921 Free PMC article.
-
Phase geometries of two-dimensional excitable waves govern self-organized morphodynamics of amoeboid cells.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):5016-21. doi: 10.1073/pnas.1218025110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479620 Free PMC article.
-
Pseudopodium activation and inhibition signals in chemotaxis by Dictyostelium discoideum amoebae.Semin Cell Biol. 1990 Apr;1(2):87-97. Semin Cell Biol. 1990. PMID: 2102388 Review.
-
Signaling pathways mediating chemotaxis in the social amoeba, Dictyostelium discoideum.Eur J Cell Biol. 2006 Sep;85(9-10):897-904. doi: 10.1016/j.ejcb.2006.06.003. Eur J Cell Biol. 2006. PMID: 16962888 Review.
Cited by
-
T cell morphodynamics reveal periodic shape oscillations in three-dimensional migration.J R Soc Interface. 2022 May;19(190):20220081. doi: 10.1098/rsif.2022.0081. Epub 2022 May 11. J R Soc Interface. 2022. PMID: 35537475 Free PMC article.
-
Extracting microtentacle dynamics of tumor cells in a non-adherent environment.Oncotarget. 2017 Dec 4;8(67):111567-111580. doi: 10.18632/oncotarget.22874. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340075 Free PMC article.
-
Time series modeling of live-cell shape dynamics for image-based phenotypic profiling.Integr Biol (Camb). 2016 Jan;8(1):73-90. doi: 10.1039/c5ib00283d. Epub 2015 Dec 11. Integr Biol (Camb). 2016. PMID: 26658688 Free PMC article.
-
From actin waves to mechanism and back: How theory aids biological understanding.Elife. 2023 Jul 10;12:e87181. doi: 10.7554/eLife.87181. Elife. 2023. PMID: 37428017 Free PMC article.
-
Regimes of wave type patterning driven by refractory actin feedback: transition from static polarization to dynamic wave behaviour.Phys Biol. 2012 Aug;9(4):046005. doi: 10.1088/1478-3975/9/4/046005. Epub 2012 Jul 11. Phys Biol. 2012. PMID: 22785332 Free PMC article.
References
-
- Wagle MA, Tranquillo RT. A self-consistent cell flux expression for simultaneous chemotaxis and contact guidance in tissues. J Math Biol. 2000;41:315–330. - PubMed
-
- Yang X, Dormann D, Munsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell. 2002;3:425–437. - PubMed
-
- Capogrosso Sansone B, Scalerandi M, Condat CA. Emergence of taxis and synergy in angiogenesis. Phys Rev Lett. 2001;87:128102. - PubMed
-
- Kassis J, Lauffenburger DA, Turner T, Wells A. Tumor invasion as dysregulated cell motility. Semin Cancer Biol. 2001;11:105–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

