Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker
- PMID: 22433855
- PMCID: PMC3351316
- DOI: 10.1074/jbc.M111.304881
Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker
Abstract
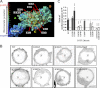
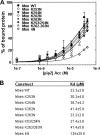
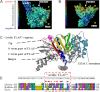
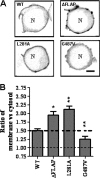
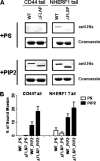
Many cellular processes depend on ERM (ezrin, moesin, and radixin) proteins mediating regulated linkage between plasma membrane and actin cytoskeleton. Although conformational activation of the ERM protein is mediated by the membrane PIP2, the known properties of the two described PIP2-binding sites do not explain activation. To elucidate the structural basis of possible mechanisms, we generated informative moesin mutations and tested three attributes: membrane localization of the expressed moesin, moesin binding to PIP2, and PIP2-induced release of moesin autoinhibition. The results demonstrate for the first time that the POCKET containing inositol 1,4,5-trisphosphate on crystal structure (the "POCKET" Lys-63, Lys-278 residues) mediates all three functions. Furthermore the second described PIP2-binding site (the "PATCH," Lys-253/Lys-254, Lys-262/Lys-263) is also essential for all three functions. In native autoinhibited ERM proteins, the POCKET is a cavity masked by an acidic linker, which we designate the "FLAP." Analysis of three mutant moesin constructs predicted to influence FLAP function demonstrated that the FLAP is a functional autoinhibitory region. Moreover, analysis of the cooperativity and stoichiometry demonstrate that the PATCH and POCKET do not bind PIP2 simultaneously. Based on our data and supporting published data, we propose a model of progressive activation of autoinhibited moesin by a single PIP2 molecule in the membrane. Initial transient binding of PIP2 to the PATCH initiates release of the FLAP, which enables transition of the same PIP2 molecule into the newly exposed POCKET where it binds stably and completes the conformational activation.
Figures


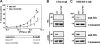

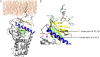

Similar articles
-
Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane.J Cell Biol. 2009 Feb 9;184(3):451-62. doi: 10.1083/jcb.200807047. J Cell Biol. 2009. PMID: 19204146 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate alters the number of attachment sites between ezrin and actin filaments: a colloidal probe study.J Biol Chem. 2014 Apr 4;289(14):9833-43. doi: 10.1074/jbc.M113.530659. Epub 2014 Feb 5. J Biol Chem. 2014. PMID: 24500715 Free PMC article.
-
Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway.J Cell Biol. 1996 Oct;135(1):37-51. doi: 10.1083/jcb.135.1.37. J Cell Biol. 1996. PMID: 8858161 Free PMC article.
-
Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins.J Biol Chem. 1999 Dec 3;274(49):34507-10. doi: 10.1074/jbc.274.49.34507. J Biol Chem. 1999. PMID: 10574907 Review. No abstract available.
-
CLIC proteins, ezrin, radixin, moesin and the coupling of membranes to the actin cytoskeleton: a smoking gun?Biochim Biophys Acta. 2014 Feb;1838(2):643-57. doi: 10.1016/j.bbamem.2013.05.025. Epub 2013 Jun 1. Biochim Biophys Acta. 2014. PMID: 23732235 Review.
Cited by
-
A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster.Mol Psychiatry. 2021 Aug;26(8):4417-4430. doi: 10.1038/s41380-019-0620-0. Epub 2019 Dec 3. Mol Psychiatry. 2021. PMID: 31796894 Free PMC article.
-
MARCKS regulates tonic and chronic active B cell receptor signaling.Leukemia. 2019 Mar;33(3):710-729. doi: 10.1038/s41375-018-0244-4. Epub 2018 Sep 12. Leukemia. 2019. PMID: 30209404
-
PI3Kα-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease.Nat Commun. 2018 Dec 19;9(1):5390. doi: 10.1038/s41467-018-07812-8. Nat Commun. 2018. PMID: 30568254 Free PMC article.
-
A bipartite NLS motif mediates the nuclear import of Drosophila moesin.Front Cell Dev Biol. 2024 Feb 21;12:1206067. doi: 10.3389/fcell.2024.1206067. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38450250 Free PMC article.
-
Potential Role of Moesin in Regulating Mast Cell Secretion.Int J Mol Sci. 2023 Jul 28;24(15):12081. doi: 10.3390/ijms241512081. Int J Mol Sci. 2023. PMID: 37569454 Free PMC article. Review.
References
-
- Hughes S. C., Fehon R. G. (2007) Understanding ERM proteins. The awesome power of genetics finally brought to bear. Curr. Opin. Cell Biol. 19, 51–56 - PubMed
-
- Niggli V., Rossy J. (2008) Ezrin/radixin/moesin, versatile controllers of signaling molecules and of the cortical cytoskeleton. Int. J. Biochem. Cell Biol. 40, 344–349 - PubMed
-
- Bretscher A., Edwards K., Fehon R. G. (2002) ERM proteins and merlin. Integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 - PubMed
-
- Fiévet B., Louvard D., Arpin M. (2007) ERM proteins in epithelial cell organization and functions. Biochim. Biophys. Acta 1773, 653–660 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

