Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis
- PMID: 22432015
- PMCID: PMC3303821
- DOI: 10.1371/journal.pone.0033360
Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis
Abstract
Background and aims: Mesenchymal stromal cells (MSCs) were shown to have immunomodulatory activity and have been applied for treating immune-mediated disorders. We compared the homing and therapeutic action of cryopreserved subcutaneous adipose tissue (AT-MSCs) and bone marrow-derived mesenchymal stromal cells (BM-MSCs) in rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis.
Methods: After colonoscopic detection of inflammation AT-MSCs or BM-MSCs were injected intraperitoneally. Colonoscopic and histologic scores were obtained. Density of collagen fibres and apoptotic rates were evaluated. Cytokine levels were measured in supernatants of colon explants. For cell migration studies MSCs and skin fibroblasts were labelled with Tc-99m or CM-DiI and injected intraperitonealy or intravenously.
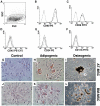
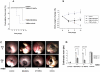
Results: Intraperitoneal injection of AT-MSCs or BM-MSCs reduced the endoscopic and histopathologic severity of colitis, the collagen deposition, and the epithelial apoptosis. Levels of TNF-α and interleukin-1β decreased, while VEGF and TGF-β did not change following cell-therapy. Scintigraphy showed that MSCs migrated towards the inflamed colon and the uptake increased from 0.5 to 24 h. Tc-99m-MSCs injected intravenously distributed into various organs, but not the colon. Cm-DiI-positive MSCs were detected throughout the colon wall 72 h after inoculation, predominantly in the submucosa and muscular layer of inflamed areas.
Conclusions: Intraperitoneally injected cryopreserved MSCs home to and engraft into the inflamed colon and ameliorate TNBS-colitis.
Conflict of interest statement
Figures





Similar articles
-
Comparison of Adipose-Derived and Bone Marrow Mesenchymal Stromal Cells in a Murine Model of Crohn's Disease.Dig Dis Sci. 2017 Jan;62(1):115-123. doi: 10.1007/s10620-016-4166-6. Epub 2016 Apr 23. Dig Dis Sci. 2017. PMID: 27107864
-
Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis.Cell Transplant. 2011;20(9):1395-408. doi: 10.3727/096368910X557245. Epub 2011 Mar 9. Cell Transplant. 2011. PMID: 21396175
-
Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.World J Gastroenterol. 2013 Aug 7;19(29):4702-17. doi: 10.3748/wjg.v19.i29.4702. World J Gastroenterol. 2013. PMID: 23922467 Free PMC article.
-
Combined effect of bone marrow derived mesenchymal stem cells and nitric oxide inducer on injured gastric mucosa in a rat model.Tissue Cell. 2016 Dec;48(6):644-652. doi: 10.1016/j.tice.2016.09.006. Epub 2016 Sep 28. Tissue Cell. 2016. PMID: 27751517 Review.
-
Review: mesenchymal stem cells and corneal reconstruction.Mol Vis. 2013 Nov 7;19:2237-43. eCollection 2013. Mol Vis. 2013. PMID: 24227919 Free PMC article. Review.
Cited by
-
Extracellular Vesicles Secreted by Mesenchymal Stromal Cells Exert Opposite Effects to Their Cells of Origin in Murine Sodium Dextran Sulfate-Induced Colitis.Front Immunol. 2021 Apr 13;12:627605. doi: 10.3389/fimmu.2021.627605. eCollection 2021. Front Immunol. 2021. PMID: 33927713 Free PMC article.
-
Role of mesenchymal stem cells in sepsis and their therapeutic potential in sepsis‑associated myopathy (Review).Int J Mol Med. 2024 Nov;54(5):92. doi: 10.3892/ijmm.2024.5416. Epub 2024 Sep 2. Int J Mol Med. 2024. PMID: 39219272 Free PMC article. Review.
-
Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects.Int J Mol Sci. 2019 Jul 12;20(14):3430. doi: 10.3390/ijms20143430. Int J Mol Sci. 2019. PMID: 31336890 Free PMC article. Review.
-
Adipose Tissue-Derived Mesenchymal Stromal Cells from Ex-Morbidly Obese Individuals Instruct Macrophages towards a M2-Like Profile In Vitro.Int J Stem Cells. 2023 Nov 30;16(4):425-437. doi: 10.15283/ijsc22172. Epub 2023 Aug 30. Int J Stem Cells. 2023. PMID: 37643763 Free PMC article.
-
Mesenchymal Stem Cells of Dental Origin-Their Potential for Antiinflammatory and Regenerative Actions in Brain and Gut Damage.Curr Neuropharmacol. 2016;14(8):914-934. doi: 10.2174/1570159x14666160121115210. Curr Neuropharmacol. 2016. PMID: 26791480 Free PMC article. Review.
References
-
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–429. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal R, Douglas JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

