Shp2 controls female body weight and energy balance by integrating leptin and estrogen signals
- PMID: 22431513
- PMCID: PMC3347413
- DOI: 10.1128/MCB.06712-11
Shp2 controls female body weight and energy balance by integrating leptin and estrogen signals
Abstract
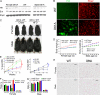
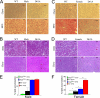
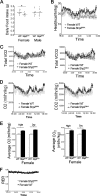
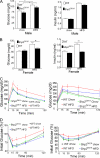
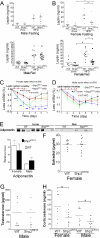
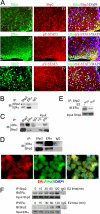
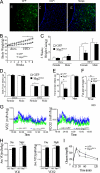
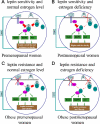
In mammals, leptin regulates food intake and energy balance mainly through the activation of LepRb in the hypothalamus, and estrogen has a leptin-like effect in the hypothalamic control of metabolism. However, it remains to be elucidated how estrogen signaling is intertwined with the leptin pathway. We show here that Shp2, a nonreceptor tyrosine phosphatase, acts to integrate leptin and estrogen signals. The expression of a dominant-active mutant (Shp2(D61A)) in forebrain neurons conferred female, but not male, transgenic mice resistance to high-fat diet (HFD)-induced obesity and liver steatosis, accompanied by improved insulin sensitivity and glucose homeostasis. Fed with either HFD or regular chow food, Shp2(D61A) female mice showed dramatically enhanced leptin sensitivity. Microinjection of Shp2(D61A)-expressing adeno-associated virus into mediobasal hypothalamus elicited a similar antiobese effect in female mice. Biochemical analyses showed a physical association of Shp2 with estrogen receptor alpha, which is necessary for the synergistic and persistent activation of Erk by leptin and estrogen. Together, these results elucidate a mechanism for the direct cross talk of leptin and estrogen signaling and offer one explanation for the propensity of postmenopausal women to develop obesity.
Figures

Similar articles
-
Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism.Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):16064-9. doi: 10.1073/pnas.0405041101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520383 Free PMC article.
-
Leptin Signaling Is Not Required for Anorexigenic Estradiol Effects in Female Mice.Endocrinology. 2016 May;157(5):1991-2001. doi: 10.1210/en.2015-1594. Epub 2016 Mar 3. Endocrinology. 2016. PMID: 26937712
-
Deletion of leptin receptors in vagal afferent neurons disrupts estrogen signaling, body weight, food intake and hormonal controls of feeding in female mice.Am J Physiol Endocrinol Metab. 2019 Apr 1;316(4):E568-E577. doi: 10.1152/ajpendo.00296.2018. Epub 2019 Feb 12. Am J Physiol Endocrinol Metab. 2019. PMID: 30753113 Free PMC article.
-
Shp2 as a therapeutic target for leptin resistance and obesity.Expert Opin Ther Targets. 2006 Feb;10(1):135-42. doi: 10.1517/14728222.10.1.135. Expert Opin Ther Targets. 2006. PMID: 16441233 Review.
-
Leptin signalling pathways in hypothalamic neurons.Cell Mol Life Sci. 2016 Apr;73(7):1457-77. doi: 10.1007/s00018-016-2133-1. Epub 2016 Jan 19. Cell Mol Life Sci. 2016. PMID: 26786898 Free PMC article. Review.
Cited by
-
STAT3 phosphorylation in central leptin resistance.Nutr Metab (Lond). 2021 Apr 13;18(1):39. doi: 10.1186/s12986-021-00569-w. Nutr Metab (Lond). 2021. PMID: 33849593 Free PMC article. Review.
-
Metabolic regulation by protein tyrosine phosphatases.J Biomed Res. 2014 May;28(3):157-68. doi: 10.7555/JBR.28.20140012. Epub 2014 Feb 28. J Biomed Res. 2014. PMID: 25013399 Free PMC article.
-
Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis.Front Med. 2012 Sep;6(3):275-9. doi: 10.1007/s11684-012-0216-4. Epub 2012 Aug 6. Front Med. 2012. PMID: 22869052 Review.
-
Modeling (not so) rare developmental disorders associated with mutations in the protein-tyrosine phosphatase SHP2.Front Cell Dev Biol. 2022 Nov 4;10:1046415. doi: 10.3389/fcell.2022.1046415. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36407105 Free PMC article. Review.
-
Control of body weight versus tumorigenesis by concerted action of leptin and estrogen.Rev Endocr Metab Disord. 2013 Dec;14(4):339-45. doi: 10.1007/s11154-013-9277-1. Rev Endocr Metab Disord. 2013. PMID: 24142297 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
