The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language
- PMID: 22426781
- PMCID: PMC3375077
- DOI: 10.1007/s10519-012-9532-3
The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language
Abstract
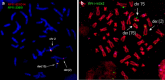
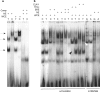
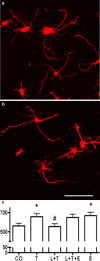
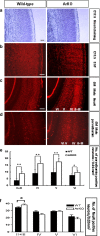
Inspired by the localization, on 15q21.2 of the CYP19A1 gene in the linkage region of speech and language disorders, and a rare translocation in a dyslexic individual that was brought to our attention, we conducted a series of studies on the properties of CYP19A1 as a candidate gene for dyslexia and related conditions. The aromatase enzyme is a member of the cytochrome P450 super family, and it serves several key functions: it catalyzes the conversion of androgens into estrogens; during early mammalian development it controls the differentiation of specific brain areas (e.g. local estrogen synthesis in the hippocampus regulates synaptic plasticity and axonal growth); it is involved in sexual differentiation of the brain; and in songbirds and teleost fishes, it regulates vocalization. Our results suggest that variations in CYP19A1 are associated with dyslexia as a categorical trait and with quantitative measures of language and speech, such as reading, vocabulary, phonological processing and oral motor skills. Variations near the vicinity of its brain promoter region altered transcription factor binding, suggesting a regulatory role in CYP19A1 expression. CYP19A1 expression in human brain correlated with the expression of dyslexia susceptibility genes such as DYX1C1 and ROBO1. Aromatase-deficient mice displayed increased cortical neuronal density and occasional cortical heterotopias, also observed in Robo1-/- mice and human dyslexic brains, respectively. An aromatase inhibitor reduced dendritic growth in cultured rat neurons. From this broad set of evidence, we propose CYP19A1 as a candidate gene for human cognitive functions implicated in reading, speech and language.
Figures


Similar articles
-
Genetic variance in a component of the language acquisition device: ROBO1 polymorphisms associated with phonological buffer deficits.Behav Genet. 2011 Jan;41(1):50-7. doi: 10.1007/s10519-010-9402-9. Epub 2010 Oct 15. Behav Genet. 2011. PMID: 20949370
-
Human speech- and reading-related genes display partially overlapping expression patterns in the marmoset brain.Brain Lang. 2014 Jun;133:26-38. doi: 10.1016/j.bandl.2014.03.007. Epub 2014 Apr 24. Brain Lang. 2014. PMID: 24769279
-
The rs3743205 SNP is important for the regulation of the dyslexia candidate gene DYX1C1 by estrogen receptor β and DNA methylation.Mol Endocrinol. 2012 Apr;26(4):619-29. doi: 10.1210/me.2011-1376. Epub 2012 Mar 1. Mol Endocrinol. 2012. PMID: 22383464 Free PMC article.
-
The human lexinome: genes of language and reading.J Commun Disord. 2008 Sep-Oct;41(5):409-20. doi: 10.1016/j.jcomdis.2008.03.003. Epub 2008 Mar 25. J Commun Disord. 2008. PMID: 18466916 Free PMC article. Review.
-
Breakthroughs in the search for dyslexia candidate genes.Trends Mol Med. 2006 Jul;12(7):333-41. doi: 10.1016/j.molmed.2006.05.007. Epub 2006 Jun 16. Trends Mol Med. 2006. PMID: 16781891 Review.
Cited by
-
Importance of copy number variants in childhood apraxia of speech and other speech sound disorders.Commun Biol. 2024 Oct 5;7(1):1273. doi: 10.1038/s42003-024-06968-y. Commun Biol. 2024. PMID: 39369109 Free PMC article.
-
Reading and language disorders: the importance of both quantity and quality.Genes (Basel). 2014 Apr 4;5(2):285-309. doi: 10.3390/genes5020285. Genes (Basel). 2014. PMID: 24705331 Free PMC article.
-
Neuroanatomical and molecular correlates of cognitive and behavioural outcomes in hypogonadal males.Metab Brain Dis. 2018 Apr;33(2):491-505. doi: 10.1007/s11011-017-0163-5. Epub 2017 Dec 11. Metab Brain Dis. 2018. PMID: 29230619 Review.
-
In-silico and in-vitro study reveals ziprasidone as a potential aromatase inhibitor against breast carcinoma.Sci Rep. 2023 Oct 2;13(1):16545. doi: 10.1038/s41598-023-43789-1. Sci Rep. 2023. PMID: 37783782 Free PMC article.
-
The genetics of reading disabilities: from phenotypes to candidate genes.Front Psychol. 2013 Jan 7;3:601. doi: 10.3389/fpsyg.2012.00601. eCollection 2012. Front Psychol. 2013. PMID: 23308072 Free PMC article.
References
-
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42.
-
- Anthoni H, Zucchelli M, Matsson H, Muller-Myhsok B, Fransson I, Schumacher J, Massinen S, Onkamo P, Warnke A, Griesemann H, Hoffmann P, Nopola-Hemmi J, Lyytinen H, Schulte-Korne G, Kere J, Nothen MM, Peyrard-Janvid M. A locus on 2p12 containing the co-regulated MRPL19 and C2ORF3 genes is associated to dyslexia. Hum Mol Genet. 2007;16(6):667–677. doi: 10.1093/hmg/ddm009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

