Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium
- PMID: 22419848
- PMCID: PMC3298421
Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium
Abstract
Purpose: To test the feasibility of altering the phenotype of umbilical cord blood mesenchymal stem cells (UCB MSCs) toward that of human corneal endothelial cells (HCEC) and to determine whether UCB MSCs can "home" to sites of corneal endothelial cell injury using an ex vivo corneal wound model.
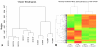
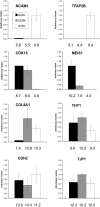
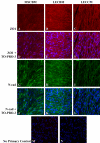
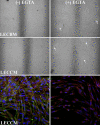
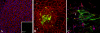
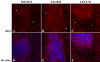
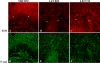
Methods: RNA was isolated and purified from UCB MSCs and HCECs. Baseline information regarding the relative gene expression of UCB MSCs and HCEC was obtained by microarray analysis. Quantitative real-time PCR (q-PCR) verified the microarray findings for a subset of genes. The ability of different culture media to direct UCB MSCs toward a more HCEC-like phenotype was tested in both tissue culture and ex vivo corneal endothelial wound models using three different media: MSC basal medium (MSCBM), a basal medium used to culture lens epithelial cells (LECBM), or lens epithelial cell-conditioned medium (LECCM). Morphology of the MSCs was observed by phase-contrast microscopy or by light microscopic observation of crystal violet-stained cells. Immunolocalization of the junction-associated proteins, zonula occludins-1 (ZO1) and N-cadherin, was visualized by fluorescence confocal microscopy. Formation of cell-cell junctions was tested by treatment with the calcium chelator, EGTA. A second microarray analysis compared gene expression between UCB MSCs grown in LECBM and LECCM to identify changes induced by the lens epithelial cell-conditioned culture medium. The ability of UCB MSCs to "home" to areas of endothelial injury was determined using ZO1 immunolocalization patterns in ex vivo corneal endothelial wounds.
Results: Baseline microarray analysis provided information regarding relative gene expression in UCB MSCs and HCECs. MSCs attached to damaged, but not intact, corneal endothelium in ex vivo corneal wounds. The morphology of MSCs was consistently altered when cells were grown in the presence of LECCM. In tissue culture and in ex vivo corneal wounds, UCB MSC treated with LECCM were elongated and formed parallel sheets of closely apposed cells. In both tissue culture and ex vivo corneal endothelial wounds, ZO1 and N-cadherin localized mainly to the cytoplasm of UCB MSCs in the presence of MSCBM. However, both proteins localized to cell borders when UCB MSCs were grown in either LECBM or LECCM. This localization was lost when extracellular calcium levels were reduced by treatment with EGTA. A second microarray analysis showed that, when UCB MSCs were grown in LECCM instead of LECBM, the relative expression of a subset of genes markedly differed, suggestive of a more HCEC-like phenotype.
Conclusions: Results indicate that UCB MSCs are able to "home" to areas of injured corneal endothelium and that the phenotype of UCB MSCs can be altered toward that of HCEC-like cells. Further study is needed to identify the specific microenvironmental conditions that would permit tissue engineering of UCB MSCs to replace damaged or diseased corneal endothelium.
Figures


Similar articles
-
Y-27632 Promotes the Repair Effect of Umbilical Cord Blood-Derived Endothelial Progenitor Cells on Corneal Endothelial Wound Healing.Cornea. 2021 Feb 1;40(2):203-214. doi: 10.1097/ICO.0000000000002560. Cornea. 2021. PMID: 33086282
-
Cultivation of an immortalized human corneal endothelial cell population and two distinct clonal subpopulations on thermo-responsive carriers.Graefes Arch Clin Exp Ophthalmol. 2008 Nov;246(11):1575-83. doi: 10.1007/s00417-008-0904-6. Epub 2008 Aug 12. Graefes Arch Clin Exp Ophthalmol. 2008. PMID: 18696098
-
Platelet-rich plasma improves the therapeutic efficacy of mesenchymal stem cells by enhancing their secretion of angiogenic factors in a combined radiation and wound injury model.Exp Dermatol. 2020 Feb;29(2):158-167. doi: 10.1111/exd.14042. Epub 2019 Nov 18. Exp Dermatol. 2020. PMID: 31560791
-
Corneal endothelial regeneration and tissue engineering.Prog Retin Eye Res. 2013 Jul;35:1-17. doi: 10.1016/j.preteyeres.2013.01.003. Epub 2013 Jan 23. Prog Retin Eye Res. 2013. PMID: 23353595 Review.
-
Repairing neural injuries using human umbilical cord blood.Mol Neurobiol. 2013 Jun;47(3):938-45. doi: 10.1007/s12035-012-8388-0. Epub 2012 Dec 30. Mol Neurobiol. 2013. PMID: 23275174 Free PMC article. Review.
Cited by
-
Synaptic Plasticity of Human Umbilical Cord Mesenchymal Stem Cell Differentiating into Neuron-like Cells In Vitro Induced by Edaravone.Stem Cells Int. 2018 Oct 28;2018:5304279. doi: 10.1155/2018/5304279. eCollection 2018. Stem Cells Int. 2018. PMID: 30510585 Free PMC article.
-
Ocular-Surface Regeneration Therapies for Eye Disorders: The State of the Art.BioTech (Basel). 2023 Jun 15;12(2):48. doi: 10.3390/biotech12020048. BioTech (Basel). 2023. PMID: 37366796 Free PMC article. Review.
-
Concise Review: Stem Cells for Corneal Wound Healing.Stem Cells. 2017 Oct;35(10):2105-2114. doi: 10.1002/stem.2667. Epub 2017 Jul 26. Stem Cells. 2017. PMID: 28748596 Free PMC article. Review.
-
Stem cell therapy in ocular pathologies in the past 20 years.World J Stem Cells. 2021 May 26;13(5):366-385. doi: 10.4252/wjsc.v13.i5.366. World J Stem Cells. 2021. PMID: 34136071 Free PMC article. Review.
-
Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair.Stem Cells Transl Med. 2018 Dec;7(12):906-917. doi: 10.1002/sctm.18-0042. Epub 2018 Sep 10. Stem Cells Transl Med. 2018. PMID: 30260581 Free PMC article.
References
-
- Rasouli M, Caraiscos VB, Slomovic AR. Efficacy of routine notification and request on reducing corneal transplantation wait times in Canada. Can J Ophthalmol. 2009;44:31–5. - PubMed
-
- Rosenbaum K, Rottler J, Steinbach R, Huber KK. Reduced availability of potential cornea donors: reasons and suggestions. Klin Monatsbl Augenheilkd. 2010;227:418–22. - PubMed
-
- Shimazaki J, Shinozaki N, Shimmura S, Holland EJ, Tsubota K. Efficacy and safety of international donor sharing: a single-center, case-controlled study on corneal transplantation. Transplantation. 2004;78:216–20. - PubMed
-
- Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23:S8–19. - PubMed
-
- Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81:629–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
