Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain
- PMID: 22412988
- PMCID: PMC3297629
- DOI: 10.1371/journal.pone.0033105
Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain
Abstract
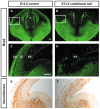
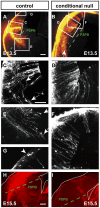
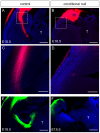
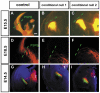
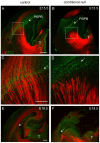
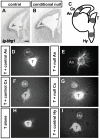
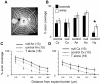
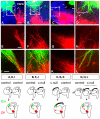
Developing thalamocortical axons traverse the subpallium to reach the cortex located in the pallium. We tested the hypothesis that descending corticofugal axons are important for guiding thalamocortical axons across the pallial-subpallial boundary, using conditional mutagenesis to assess the effects of blocking corticofugal axonal development without disrupting thalamus, subpallium or the pallial-subpallial boundary. We found that thalamic axons still traversed the subpallium in topographic order but did not cross the pallial-subpallial boundary. Co-culture experiments indicated that the inability of thalamic axons to cross the boundary was not explained by mutant cortex developing a long-range chemorepulsive action on thalamic axons. On the contrary, cortex from conditional mutants retained its thalamic axonal growth-promoting activity and continued to express Nrg-1, which is responsible for this stimulatory effect. When mutant cortex was replaced with control cortex, corticofugal efferents were restored and thalamic axons from conditional mutants associated with them and crossed the pallial-subpallial boundary. Our study provides the most compelling evidence to date that cortical efferents are required to guide thalamocortical axons across the pallial-subpallial boundary, which is otherwise hostile to thalamic axons. These results support the hypothesis that thalamic axons grow from subpallium to cortex guided by cortical efferents, with stimulation from diffusible cortical growth-promoting factors.
Conflict of interest statement
Figures

Similar articles
-
Defects in reciprocal projections between the thalamus and cerebral cortex in the early development of Fezl-deficient mice.J Comp Neurol. 2007 Jul 20;503(3):454-65. doi: 10.1002/cne.21401. J Comp Neurol. 2007. PMID: 17503485
-
Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other.J Comp Neurol. 2002 May 20;447(1):8-17. doi: 10.1002/cne.10219. J Comp Neurol. 2002. PMID: 11967891
-
Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain.Eur J Neurosci. 2012 May;35(10):1573-85. doi: 10.1111/j.1460-9568.2012.08119.x. Eur J Neurosci. 2012. PMID: 22607003 Free PMC article. Review.
-
Pax6 is required for the normal development of the forebrain axonal connections.Development. 2002 Nov;129(21):5041-52. doi: 10.1242/dev.129.21.5041. Development. 2002. PMID: 12397112
-
Development and Evolution of Thalamocortical Connectivity.Cold Spring Harb Perspect Biol. 2024 Jan 2;16(1):a041503. doi: 10.1101/cshperspect.a041503. Cold Spring Harb Perspect Biol. 2024. PMID: 38167425 Review.
Cited by
-
Ablation of CNTN2+ Pyramidal Neurons During Development Results in Defects in Neocortical Size and Axonal Tract Formation.Front Cell Neurosci. 2019 Nov 1;13:454. doi: 10.3389/fncel.2019.00454. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31749685 Free PMC article.
-
Linx mediates interaxonal interactions and formation of the internal capsule.Neuron. 2014 Jul 2;83(1):93-103. doi: 10.1016/j.neuron.2014.05.020. Epub 2014 Jun 12. Neuron. 2014. PMID: 24930700 Free PMC article.
-
Mutation of the BiP/GRP78 gene causes axon outgrowth and fasciculation defects in the thalamocortical connections of the mammalian forebrain.J Comp Neurol. 2013 Feb 15;521(3):677-96. doi: 10.1002/cne.23199. J Comp Neurol. 2013. PMID: 22821687 Free PMC article.
-
Chromatin remodeler Arid1a regulates subplate neuron identity and wiring of cortical connectivity.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2100686118. doi: 10.1073/pnas.2100686118. Proc Natl Acad Sci U S A. 2021. PMID: 34011608 Free PMC article.
-
Trans-Axonal Signaling in Neural Circuit Wiring.Int J Mol Sci. 2020 Jul 21;21(14):5170. doi: 10.3390/ijms21145170. Int J Mol Sci. 2020. PMID: 32708320 Free PMC article. Review.
References
-
- Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. - PubMed
-
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. - PubMed
-
- Molnar Z, Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991;351:475–477. - PubMed
-
- Tuttle R, Nakagawa Y, Johnson JE, O'Leary DD. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

