Migration of Th1 lymphocytes is regulated by CD152 (CTLA-4)-mediated signaling via PI3 kinase-dependent Akt activation
- PMID: 22412835
- PMCID: PMC3295805
- DOI: 10.1371/journal.pone.0031391
Migration of Th1 lymphocytes is regulated by CD152 (CTLA-4)-mediated signaling via PI3 kinase-dependent Akt activation
Abstract
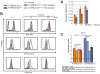
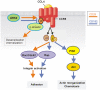
Efficient adaptive immune responses require the localization of T lymphocytes in secondary lymphoid organs and inflamed tissues. To achieve correct localization of T lymphocytes, the migration of these cells is initiated and directed by adhesion molecules and chemokines. It has recently been shown that the inhibitory surface molecule CD152 (CTLA-4) initiates Th cell migration, but the molecular mechanism underlying this effect remains to be elucidated. Using CD4 T lymphocytes derived from OVA-specific TCR transgenic CD152-deficient and CD152-competent mice, we demonstrate that chemokine-triggered signal transduction is differentially regulated by CD152 via phosphoinositide 3-kinase (PI3K)-dependent activation of protein kinase B (PKB/Akt). In the presence of CD152 signaling, the chemoattractant CCL4 selectively induces the full activation of Akt via phosphorylation at threonine 308 and serine 473 in pro-inflammatory Th lymphocytes expressing the cognate chemokine receptor CCR5. Akt signals lead to cytoskeleton rearrangements, which are indispensable for migration. Therefore, this novel Akt-modulating function of CD152 signals affecting T cell migration demonstrates that boosting CD152 or its down-stream signal transduction could aid therapies aimed at sensitizing T lymphocytes for optimal migration, thus contributing to a precise and effective immune response.
Conflict of interest statement
Figures


Similar articles
-
CD152 (CTLA-4) determines CD4 T cell migration in vitro and in vivo.PLoS One. 2009 May 27;4(5):e5702. doi: 10.1371/journal.pone.0005702. PLoS One. 2009. PMID: 19479036 Free PMC article.
-
27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes.Toxicol Appl Pharmacol. 2014 Feb 1;274(3):462-70. doi: 10.1016/j.taap.2013.12.007. Epub 2013 Dec 24. Toxicol Appl Pharmacol. 2014. PMID: 24370436
-
CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells.J Immunol. 2002 Mar 15;168(6):2729-36. doi: 10.4049/jimmunol.168.6.2729. J Immunol. 2002. PMID: 11884439
-
Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration.Ann N Y Acad Sci. 2010 Jan;1183:149-57. doi: 10.1111/j.1749-6632.2009.05134.x. Ann N Y Acad Sci. 2010. PMID: 20146713 Free PMC article. Review.
-
The role of CTLA-4 in the regulation of T cell immune responses.Immunol Cell Biol. 1999 Feb;77(1):1-10. doi: 10.1046/j.1440-1711.1999.00795.x. Immunol Cell Biol. 1999. PMID: 10101680 Review.
Cited by
-
Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology.Molecules. 2019 Mar 26;24(6):1190. doi: 10.3390/molecules24061190. Molecules. 2019. PMID: 30917623 Free PMC article. Review.
-
T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells.Front Immunol. 2020 Aug 18;11:2025. doi: 10.3389/fimmu.2020.02025. eCollection 2020. Front Immunol. 2020. PMID: 32973809 Free PMC article. Review.
-
Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types.Front Immunol. 2020 Dec 15;11:608024. doi: 10.3389/fimmu.2020.608024. eCollection 2020. Front Immunol. 2020. PMID: 33384695 Free PMC article. Review.
-
Genetics of primary sclerosing cholangitis and pathophysiological implications.Nat Rev Gastroenterol Hepatol. 2017 May;14(5):279-295. doi: 10.1038/nrgastro.2016.154. Epub 2017 Mar 15. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28293027 Review.
-
Molecular mechanisms of T cell co-stimulation and co-inhibition.Nat Rev Immunol. 2013 Apr;13(4):227-42. doi: 10.1038/nri3405. Epub 2013 Mar 8. Nat Rev Immunol. 2013. PMID: 23470321 Free PMC article. Review.
References
-
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. - PubMed
-
- Weninger W, von Andrian UH. Chemokine regulation of naive T cell traffic in health and disease. Semin Immunol. 2003;15:257–270. - PubMed
-
- Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med. 2000;191:61–76. - PMC - PubMed
-
- Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. - PubMed
-
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

