mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas
- PMID: 22409888
- PMCID: PMC3443560
- DOI: 10.1158/1078-0432.CCR-11-2824
mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas
Abstract
Purpose: The incidence of head and neck squamous cell carcinomas (HNSCC) associated with human papillomavirus (HPV) infection has increased over the past decades in the United States. We aimed at examining the global impact of HPV-associated HNSCC and whether the established key role of mTOR activation in HNSCC is also observed in HPV(+) HNSCC lesions, thereby providing novel treatment options for HPV-associated HNSCC patients.
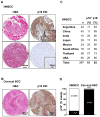
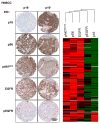
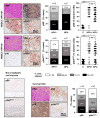
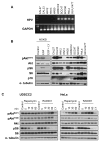
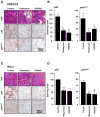
Experimental design: An international HNSCC tissue microarray (TMA) was used to analyze the expression of p16(INK4A), a surrogate for HPV infection, and Akt-mTOR pathway activation. Results were confirmed in a large collection of HPV(-) and HPV(+) HNSCC cases and in a cervical cancer (CCSCC) TMA. Observations were validated in HNSCC and CCSCC-derived cell lines, which were xenografted into immunodeficient mice for tumorigenesis assays.
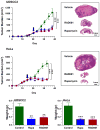
Results: Approximately 20% of all HNSCC lesions could be classified as HPV(+), irrespective of their country of origin. mTOR pathway activation was observed in most HPV(+) HNSCC and CCSCC lesions and cell lines. The preclinical efficacy of mTOR inhibition by rapamycin and RAD001 was explored in HPV(+) HNSCC and CCSCC tumor xenografts. Both mTOR inhibitors effectively decreased mTOR activity in vivo and caused a remarkable decrease in tumor burden. These results emphasize the emerging global impact of HPV-related HNSCCs and indicate that the activation of the mTOR pathway is a widespread event in both HPV(-) and HPV-associated HNSCC and CCSCC lesions.
Conclusions: The emerging results may provide a rationale for the clinical evaluation of mTOR inhibitors as a molecular targeted approach for the treatment of HPV-associated malignancies.
©2012 AACR.
Figures
Similar articles
-
Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3.Cancer Prev Res (Phila). 2015 Mar;8(3):197-207. doi: 10.1158/1940-6207.CAPR-14-0348. Epub 2015 Feb 13. Cancer Prev Res (Phila). 2015. PMID: 25681087 Free PMC article.
-
Absence of human papillomavirus infection and activation of PI3K-AKT pathway in cervical clear cell carcinoma.Int J Gynecol Cancer. 2013 Jul;23(6):1084-91. doi: 10.1097/IGC.0b013e3182981bdc. Int J Gynecol Cancer. 2013. PMID: 23792604
-
mTOR inhibition as an adjuvant therapy in a metastatic model of HPV+ HNSCC.Oncotarget. 2016 Apr 26;7(17):24228-41. doi: 10.18632/oncotarget.8286. Oncotarget. 2016. PMID: 27015118 Free PMC article.
-
PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer.Biochem Pharmacol. 2020 Feb;172:113729. doi: 10.1016/j.bcp.2019.113729. Epub 2019 Nov 27. Biochem Pharmacol. 2020. PMID: 31785230 Review.
-
Therapeutic strategies of different HPV status in Head and Neck Squamous Cell Carcinoma.Int J Biol Sci. 2021 Mar 10;17(4):1104-1118. doi: 10.7150/ijbs.58077. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867833 Free PMC article. Review.
Cited by
-
Current insights into the regulation of programmed cell death by TP53 mutation in cancer.Front Oncol. 2022 Oct 13;12:1023427. doi: 10.3389/fonc.2022.1023427. eCollection 2022. Front Oncol. 2022. PMID: 36313700 Free PMC article. Review.
-
Sirolimus reduces T cell cycling, immune checkpoint marker expression, and HIV-1 DNA in people with HIV.Cell Rep Med. 2024 Oct 15;5(10):101745. doi: 10.1016/j.xcrm.2024.101745. Epub 2024 Sep 24. Cell Rep Med. 2024. PMID: 39321793 Free PMC article.
-
Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3.Cancer Prev Res (Phila). 2015 Mar;8(3):197-207. doi: 10.1158/1940-6207.CAPR-14-0348. Epub 2015 Feb 13. Cancer Prev Res (Phila). 2015. PMID: 25681087 Free PMC article.
-
mTOR downstream effectors, 4EBP1 and eIF4E, are overexpressed and associated with HPV status in precancerous lesions and carcinomas of the uterine cervix.Oncol Lett. 2016 Nov;12(5):3234-3240. doi: 10.3892/ol.2016.5056. Epub 2016 Aug 29. Oncol Lett. 2016. PMID: 27899988 Free PMC article.
-
ER maleate is a novel anticancer agent in oral cancer: implications for cancer therapy.Oncotarget. 2016 Mar 29;7(13):17162-81. doi: 10.18632/oncotarget.7751. Oncotarget. 2016. PMID: 26934445 Free PMC article.
References
-
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–5. - PubMed
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. - PubMed
-
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. Lyon; 2005.
-
- Warnakulasuriya S. Causes of oral cancer--an appraisal of controversies. Br Dent J. 2009;207:471–5. - PubMed
-
- de Villiers EM, Weidauer H, Otto H, zur Hausen H. Papillomavirus DNA in human tongue carcinomas. Int J Cancer. 1985;36:575–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

