Binding of Lassa virus perturbs extracellular matrix-induced signal transduction via dystroglycan
- PMID: 22405130
- PMCID: PMC3869547
- DOI: 10.1111/j.1462-5822.2012.01784.x
Binding of Lassa virus perturbs extracellular matrix-induced signal transduction via dystroglycan
Abstract
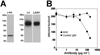
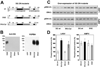
The arenavirus Lassa virus (LASV) causes a severe haemorrhagic fever with high mortality in man. The cellular receptor for LASV is dystroglycan (DG). DG is a ubiquitous receptor for extracellular matrix (ECM) proteins, which cooperates with β1 integrins to control cell-matrix interactions. Here, we investigated whether LASV binding to DG triggers signal transduction, mimicking the natural ligands. Engagement of DG by LASV resulted in the recruitment of the adaptor protein Grb2 and the protein kinase MEK1 by the cytoplasmic domain of DG without activating the MEK/ERK pathway, indicating assembly of an inactive signalling complex. LASV binding to cells however affected the activation of the MEK/ERK pathway via α6β1 integrins. The virus-induced perturbation of α6β1 integrin signalling critically depended on high-affinity LASV binding to DG and DG's cytoplasmic domain, indicating that LASV-receptor binding perturbed signalling cross-talk between DG and β1 integrins.
© 2012 Blackwell Publishing Ltd.
Figures





Similar articles
-
Lassa Fever Virus Binds Matriglycan-A Polymer of Alternating Xylose and Glucuronate-On α-Dystroglycan.Viruses. 2021 Aug 25;13(9):1679. doi: 10.3390/v13091679. Viruses. 2021. PMID: 34578260 Free PMC article. Review.
-
Lassa Virus Cell Entry via Dystroglycan Involves an Unusual Pathway of Macropinocytosis.J Virol. 2016 Jun 24;90(14):6412-6429. doi: 10.1128/JVI.00257-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147735 Free PMC article.
-
Axl Can Serve as Entry Factor for Lassa Virus Depending on the Functional Glycosylation of Dystroglycan.J Virol. 2018 Feb 12;92(5):e01613-17. doi: 10.1128/JVI.01613-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237830 Free PMC article.
-
Dynamic Dystroglycan Complexes Mediate Cell Entry of Lassa Virus.mBio. 2019 Mar 26;10(2):e02869-18. doi: 10.1128/mBio.02869-18. mBio. 2019. PMID: 30914516 Free PMC article.
-
Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction.Virology. 2009 May 10;387(2):245-9. doi: 10.1016/j.virol.2009.02.042. Epub 2009 Mar 25. Virology. 2009. PMID: 19324387 Review.
Cited by
-
Regulation of Stress-Activated Kinases in Response to Tacaribe Virus Infection and Its Implications for Viral Replication.Viruses. 2022 Sep 12;14(9):2018. doi: 10.3390/v14092018. Viruses. 2022. PMID: 36146824 Free PMC article.
-
The search for animal models for Lassa fever vaccine development.Expert Rev Vaccines. 2013 Jan;12(1):71-86. doi: 10.1586/erv.12.139. Expert Rev Vaccines. 2013. PMID: 23256740 Free PMC article. Review.
-
Transcriptome analysis of human peripheral blood mononuclear cells exposed to Lassa virus and to the attenuated Mopeia/Lassa reassortant 29 (ML29), a vaccine candidate.PLoS Negl Trop Dis. 2013 Sep 12;7(9):e2406. doi: 10.1371/journal.pntd.0002406. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24069471 Free PMC article.
-
Lassa Fever Virus Binds Matriglycan-A Polymer of Alternating Xylose and Glucuronate-On α-Dystroglycan.Viruses. 2021 Aug 25;13(9):1679. doi: 10.3390/v13091679. Viruses. 2021. PMID: 34578260 Free PMC article. Review.
-
Lassa Virus Cell Entry via Dystroglycan Involves an Unusual Pathway of Macropinocytosis.J Virol. 2016 Jun 24;90(14):6412-6429. doi: 10.1128/JVI.00257-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147735 Free PMC article.
References
-
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. - PubMed
-
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovicio SA, Satz JS, et al. LARGE can functionally bypass alpha-dystrolycan glycosylation defects in distinct congential muscular dystrophy. Nat Med. 2004;10:696–703. - PubMed
-
- Borrow P, Oldstone MB. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

