Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes
- PMID: 22399299
- PMCID: PMC3340232
- DOI: 10.1074/jbc.M111.297861
Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes
Abstract
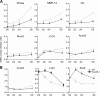
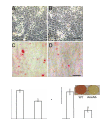
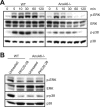
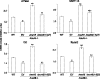
Annexin A6 (AnxA6) is highly expressed in hypertrophic and terminally differentiated growth plate chondrocytes. Rib chondrocytes isolated from newborn AnxA6-/- mice showed delayed terminal differentiation as indicated by reduced terminal differentiation markers, including alkaline phosphatase, matrix metalloproteases-13, osteocalcin, and runx2, and reduced mineralization. Lack of AnxA6 in chondrocytes led to a decreased intracellular Ca(2+) concentration and protein kinase C α (PKCα) activity, ultimately resulting in reduced extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) activities. The 45 C-terminal amino acids of AnxA6 (AnxA6(1-627)) were responsible for the direct binding of AnxA6 to PKCα. Consequently, transfection of AnxA6-/- chondrocytes with full-length AnxA6 rescued the reduced expression of terminal differentiation markers, whereas transfection of AnxA6-/- chondrocytes with AnxA6(1-627) did not or only partially rescued the decreased mRNA levels of terminal differentiation markers. In addition, lack of AnxA6 in matrix vesicles, which initiate the mineralization process in growth plate cartilage, resulted in reduced alkaline phosphatase activity and Ca(2+) and inorganic phosphate (P(i)) content and the inability to form hydroxyapatite-like crystals in vitro. Histological analysis of femoral, tibial, and rib growth plates from newborn mice revealed that the hypertrophic zone of growth plates from newborn AnxA6-/- mice was reduced in size. In addition, reduced mineralization was evident in the hypertrophic zone of AnxA6-/- growth plate cartilage, although apoptosis was not altered compared with wild type growth plates. In conclusion, AnxA6 via its stimulatory actions on PKCα and its role in mediating Ca(2+) flux across membranes regulates terminal differentiation and mineralization events of chondrocytes.
Figures




Similar articles
-
Depletion of annexin A5, annexin A6, and collagen X causes no gross changes in matrix vesicle-mediated mineralization, but lack of collagen X affects hematopoiesis and the Th1/Th2 response.J Bone Miner Res. 2012 Nov;27(11):2399-412. doi: 10.1002/jbmr.1682. J Bone Miner Res. 2012. PMID: 22692895
-
Annexin A6 interacts with p65 and stimulates NF-κB activity and catabolic events in articular chondrocytes.Arthritis Rheum. 2013 Dec;65(12):3120-9. doi: 10.1002/art.38182. Arthritis Rheum. 2013. PMID: 24022118
-
The role of pyrophosphate/phosphate homeostasis in terminal differentiation and apoptosis of growth plate chondrocytes.Bone. 2010 Sep;47(3):657-65. doi: 10.1016/j.bone.2010.06.018. Epub 2010 Jun 25. Bone. 2010. PMID: 20601283 Free PMC article.
-
[Mineralization of cartilage in growth plate].Clin Calcium. 2014 Feb;24(2):177-84. Clin Calcium. 2014. PMID: 24473350 Review. Japanese.
-
Annexin A6-A multifunctional scaffold in cell motility.Cell Adh Migr. 2017 May 4;11(3):288-304. doi: 10.1080/19336918.2016.1268318. Epub 2017 Jan 6. Cell Adh Migr. 2017. PMID: 28060548 Free PMC article. Review.
Cited by
-
Altered hepatic glucose homeostasis in AnxA6-KO mice fed a high-fat diet.PLoS One. 2018 Aug 15;13(8):e0201310. doi: 10.1371/journal.pone.0201310. eCollection 2018. PLoS One. 2018. PMID: 30110341 Free PMC article.
-
Reduced annexin A6 expression promotes the degradation of activated epidermal growth factor receptor and sensitizes invasive breast cancer cells to EGFR-targeted tyrosine kinase inhibitors.Mol Cancer. 2013 Dec 19;12(1):167. doi: 10.1186/1476-4598-12-167. Mol Cancer. 2013. PMID: 24354805 Free PMC article.
-
Annexins A2, A6 and Fetuin-A Affect the Process of Mineralization in Vesicles Derived from Human Osteoblastic hFOB 1.19 and Osteosarcoma Saos-2 Cells.Int J Mol Sci. 2021 Apr 13;22(8):3993. doi: 10.3390/ijms22083993. Int J Mol Sci. 2021. PMID: 33924370 Free PMC article.
-
Proteomic Analysis of the Peri-Infarct Area after Human Umbilical Cord Mesenchymal Stem Cell Transplantation in Experimental Stroke.Aging Dis. 2016 Oct 1;7(5):623-634. doi: 10.14336/AD.2016.0121. eCollection 2016 Oct. Aging Dis. 2016. PMID: 27699085 Free PMC article.
-
Fluid Shear Stress Regulates Osteogenic Differentiation via AnnexinA6-Mediated Autophagy in MC3T3-E1 Cells.Int J Mol Sci. 2022 Dec 11;23(24):15702. doi: 10.3390/ijms232415702. Int J Mol Sci. 2022. PMID: 36555344 Free PMC article.
References
-
- Gerke V., Moss S. E. (2002) Annexins: from structure to function. Physiol. Rev. 82, 331–371 - PubMed
-
- Geisow M. J., Walker J. H., Boustead C., Taylor W. (1988) in Molecular Mechanisms in Secretion (Thorn N. A., Traiman M., Peterson O. H., eds) pp. 598–608, Munksgaard, Copenhagen
-
- Lefebvre V., Smits P. (2005) Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today 75, 200–212 - PubMed
-
- Shimo T., Koyama E., Sugito H., Wu C., Shimo S., Pacifici M. (2005) Retinoid signaling regulates CTGF expression in hypertrophic chondrocytes with differential involvement of MAP kinases. J. Bone Miner. Res. 20, 867–877 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

