A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa
- PMID: 22395672
- PMCID: PMC3360837
- DOI: 10.1097/QAI.0b013e318251aedd
A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa
Abstract
Background: The optimal approach to HIV-associated Kaposi sarcoma (HIV-KS) in sub-Saharan Africa is unknown. With large-scale rollout of highly active antiretroviral therapy (HAART) in South Africa, we hypothesized that survival in HIV-KS would improve and administration of chemotherapy in addition to HAART would be feasible and improve KS-specific outcomes.
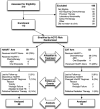
Methods: We conducted a randomized, controlled, open-label trial with intention-to-treat analysis. Treatment-naive patients from King Edward VIII Hospital, Durban, South Africa, a public-sector tertiary referral center, with HIV-KS, but no symptomatic visceral disease or fungating lesions requiring urgent chemotherapy, were randomized to HAART alone or HAART and chemotherapy (CXT). HAART arm received stavudine, lamivudine, and nevirapine (Triomune; CXT arm received Triomune plus bleomycin, doxorubicin, and vincristine every 3 weeks. When bleomycin, doxorubicin, and vincristine were not available, oral etoposide (50-100 mg for 1-21 days of a 28-day cycle) was substituted. Primary outcome was overall KS response using AIDS Clinical Trial Group criteria 12 months after HAART initiation. Secondary comparisons included time to response, progression-free survival, overall survival, adverse events, HIV control, CD4 reconstitution, adherence, and quality of life.
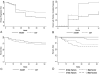
Results: Fifty-nine subjects were randomized to HAART and 53 to CXT; 12-month overall KS response was 39% in the HAART arm and 66% in the CXT arm (difference, 27%; 95% confidence interval, 9%-43%; P = 0.005). At 12 months, 77% were alive (no survival difference between arms; P = 0.49), 82% had HIV viral load <50 copies per milliliter without difference between the arms (P = 0.47); CD4 counts and quality-of-life measures improved in all patients.
Conclusions: HAART with chemotherapy produced higher overall KS response over 12 months, whereas HAART alone provided similar improvement in survival and select measures of morbidity. In Africa, with high prevalence of HIV and human herpes virus-8 and limited resources, HAART alone provides important benefit in patients with HIV-KS.
Conflict of interest statement
No other conflicts reported.
Figures
Similar articles
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014 Aug 13;8(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25221796 Free PMC article. Review.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014;(9):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25313415 Review.
-
Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi.J Int AIDS Soc. 2015 May 29;18(1):19929. doi: 10.7448/IAS.18.1.19929. eCollection 2015. J Int AIDS Soc. 2015. PMID: 26028156 Free PMC article.
-
As-Needed Vs Immediate Etoposide Chemotherapy in Combination With Antiretroviral Therapy for Mild-to-Moderate AIDS-Associated Kaposi Sarcoma in Resource-Limited Settings: A5264/AMC-067 Randomized Clinical Trial.Clin Infect Dis. 2018 Jul 2;67(2):251-260. doi: 10.1093/cid/ciy044. Clin Infect Dis. 2018. PMID: 29365083 Free PMC article. Clinical Trial.
-
Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial.Lancet. 2020 Apr 11;395(10231):1195-1207. doi: 10.1016/S0140-6736(19)33222-2. Epub 2020 Mar 5. Lancet. 2020. PMID: 32145827 Free PMC article. Clinical Trial.
Cited by
-
Patient reported outcome instruments used in clinical trials of HIV-infected adults on NNRTI-based therapy: a 10-year review.Health Qual Life Outcomes. 2013 Oct 3;11:164. doi: 10.1186/1477-7525-11-164. Health Qual Life Outcomes. 2013. PMID: 24090055 Free PMC article. Review.
-
Treatment outcomes of AIDS-associated Kaposi's sarcoma under a routine antiretroviral therapy program in Lilongwe, Malawi: bleomycin/vincristine compared to vincristine monotherapy.PLoS One. 2014 Mar 14;9(3):e91020. doi: 10.1371/journal.pone.0091020. eCollection 2014. PLoS One. 2014. PMID: 24632813 Free PMC article.
-
Suspected metastatic adrenocortical carcinoma revealing as pulmonary Kaposi sarcoma in adrenal Cushing's syndrome.BMC Endocr Disord. 2014 Jul 30;14:63. doi: 10.1186/1472-6823-14-63. BMC Endocr Disord. 2014. PMID: 25077599 Free PMC article.
-
The clinical characteristics of 80 cases of acquired immunodeficiency syndrome-associated Kaposi's sarcoma in Xinjiang Autonomous Region and the effect of different treatments on the prognosis.Int J Clin Exp Med. 2015 Oct 15;8(10):18697-704. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26770484 Free PMC article.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014 Aug 13;8(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25221796 Free PMC article. Review.
References
-
- Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21(1):S34–41. - PubMed
-
- Mosam A, Carrara H, Shaik F, et al. Increasing incidence of Kaposi's sarcoma in black South Africans in KwaZulu-Natal, South Africa (1983-2006) Int J STD AIDS. 2009 Aug;20(8):553–556. - PubMed
-
- Dedicoat M, Vaithilingum M, Newton R. Treatment of Kaposi's sarcoma in HIV-1 infected individuals with emphasis on resource poor settings. Cochrane Database Syst Rev. 2003;(3):CD003256. - PubMed
-
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodeficience humaine. AIDS. 2000;14(8):987–993. - PubMed
-
- Cattelan AM, Calabro ML, De Rossi A, et al. Long-term clinical outcome of AIDS-related Kaposi's sarcoma during highly active antiretroviral therapy. Int J Oncol. 2005 Sep;27(3):779–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

