Ribosomal protein S14 unties the MDM2-p53 loop upon ribosomal stress
- PMID: 22391559
- PMCID: PMC3736832
- DOI: 10.1038/onc.2012.63
Ribosomal protein S14 unties the MDM2-p53 loop upon ribosomal stress
Abstract
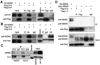
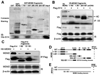
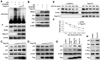
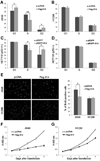
The MDM2-p53 feedback loop is crucially important for restricting p53 level and activity during normal cell growth and proliferation, and is thus subjected to dynamic regulation in order for cells to activate p53 upon various stress signals. Several ribosomal proteins, such as RPL11, RPL5, RPL23, RPL26 or RPS7, have been shown to have a role in regulation of this feedback loop in response to ribosomal stress. Here, we identify another ribosomal protein S14, which is highly associated with 5q-syndrome, as a novel activator of p53 by inhibiting MDM2 activity. We found that RPS14, but not RPS19, binds to the central acidic domain of MDM2, similar to RPL5 and RPL23, and inhibits its E3 ubiquitin ligase activity toward p53. This RPS14-MDM2 binding was induced upon ribosomal stress caused by actinomycin D or mycophenolic acid. Overexpression of RPS14, but not RPS19, elevated p53 level and activity, leading to G1 or G2 arrest. Conversely, knockdown of RPS14 alleviated p53 induction by these two reagents. Interestingly, knockdown of either RPS14 or RPS19 caused a ribosomal stress that led to p53 activation, which was impaired by further knocking down the level of RPL11 or RPL5. Together, our results demonstrate that RPS14 and RPS19 have distinct roles in regulating the MDM2-p53 feedback loop in response to ribosomal stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop.Oncogene. 2013 May 30;32(22):2782-91. doi: 10.1038/onc.2012.289. Epub 2012 Jul 9. Oncogene. 2013. PMID: 22777350 Free PMC article.
-
Regulation of the MDM2-p53 pathway by the nucleolar protein CSIG in response to nucleolar stress.Sci Rep. 2016 Nov 4;6:36171. doi: 10.1038/srep36171. Sci Rep. 2016. PMID: 27811966 Free PMC article.
-
Mice with a Mutation in the Mdm2 Gene That Interferes with MDM2/Ribosomal Protein Binding Develop a Defect in Erythropoiesis.PLoS One. 2016 Apr 4;11(4):e0152263. doi: 10.1371/journal.pone.0152263. eCollection 2016. PLoS One. 2016. PMID: 27042854 Free PMC article.
-
Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability.Oncotarget. 2014 Feb 28;5(4):860-71. doi: 10.18632/oncotarget.1784. Oncotarget. 2014. PMID: 24658219 Free PMC article. Review.
-
The RP-Mdm2-p53 pathway and tumorigenesis.Oncotarget. 2011 Mar;2(3):234-8. doi: 10.18632/oncotarget.228. Oncotarget. 2011. PMID: 21406728 Free PMC article. Review.
Cited by
-
Differential expression of RBM5 and KRAS in pancreatic ductal adenocarcinoma and their association with clinicopathological features.Oncol Lett. 2013 Mar;5(3):1000-1004. doi: 10.3892/ol.2012.1080. Epub 2012 Dec 17. Oncol Lett. 2013. PMID: 23425895 Free PMC article.
-
SPIN1 promotes tumorigenesis by blocking the uL18 (universal large ribosomal subunit protein 18)-MDM2-p53 pathway in human cancer.Elife. 2018 Mar 16;7:e31275. doi: 10.7554/eLife.31275. Elife. 2018. PMID: 29547122 Free PMC article.
-
Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy.Signal Transduct Target Ther. 2021 Aug 30;6(1):323. doi: 10.1038/s41392-021-00728-8. Signal Transduct Target Ther. 2021. PMID: 34462428 Free PMC article. Review.
-
Ribosomopathies: mechanisms of disease.Clin Med Insights Blood Disord. 2014 Aug 14;7:7-16. doi: 10.4137/CMBD.S16952. eCollection 2014. Clin Med Insights Blood Disord. 2014. PMID: 25512719 Free PMC article. Review.
-
Ribosomal protein L34 promotes the proliferation, invasion and metastasis of pancreatic cancer cells.Oncotarget. 2016 Dec 20;7(51):85259-85272. doi: 10.18632/oncotarget.13269. Oncotarget. 2016. PMID: 27845896 Free PMC article.
References
-
- Sharpless NE, DePinho RA. p53: good cop/bad cop. Cell. 2002;110(1):9–12. - PubMed
-
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. - PubMed
-
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes & development. 1993;7(7A):1126–1132. - PubMed
-
- Juven T, Barak Y, Zauberman A, George DL, Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993;8(12):3411–3416. - PubMed
-
- Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362(6423):857–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

