Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway
- PMID: 22389383
- PMCID: PMC3433057
- DOI: 10.1530/ERC-11-0368
Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway
Abstract
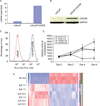
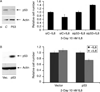
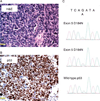
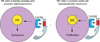
Small cell neuroendocrine carcinoma (SCNC) of the prostate is a variant form of prostate cancer that occurs de novo or as a recurrent tumor in patients who received hormonal therapy for prostatic adenocarcinoma. It is composed of pure neuroendocrine (NE) tumor cells, but unlike the scattered NE cells in benign prostate and adenocarcinoma that are quiescent, the NE cells in SCNC are highly proliferative and aggressive, causing death in months. In this study, we provide evidence that interleukin 8 (IL8)-CXCR2-P53 (TP53) signaling pathway keeps the NE cells of benign prostate and adenocarcinoma in a quiescent state normally. While P53 appears to be wild-type in the NE cells of benign prostate and adenocarcinoma, immunohistochemical studies show that the majority of the NE tumor cells in SCNC are positive for nuclear p53, suggesting that the p53 is mutated. This observation is confirmed by sequencing of genomic DNA showing p53 mutation in five of seven cases of SCNC. Our results support the hypothesis that p53 mutation leads to inactivation of the IL8-CXCR2-p53 signaling pathway, resulting in the loss of an important growth inhibitory mechanism and the hyper-proliferation of NE cells in SCNC. Therefore, we have identified potential cells of origin and a molecular target for prostatic SCNC that are very different from those of conventional adenocarcinoma, which explains SCNC's distinct biology and the clinical observation that it does not respond to hormonal therapy targeting androgen receptor signaling, which produces short-term therapeutic effects in nearly all patients with prostatic adenocarcinoma.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

Similar articles
-
p53 Mutation Directs AURKA Overexpression via miR-25 and FBXW7 in Prostatic Small Cell Neuroendocrine Carcinoma.Mol Cancer Res. 2015 Mar;13(3):584-91. doi: 10.1158/1541-7786.MCR-14-0277-T. Epub 2014 Dec 15. Mol Cancer Res. 2015. PMID: 25512615 Free PMC article.
-
Androgen-deprivation therapy-induced aggressive prostate cancer with neuroendocrine differentiation.Asian J Androl. 2014 Jul-Aug;16(4):541-4. doi: 10.4103/1008-682X.123669. Asian J Androl. 2014. PMID: 24589459 Free PMC article. Review.
-
PC3 is a cell line characteristic of prostatic small cell carcinoma.Prostate. 2011 Nov;71(15):1668-79. doi: 10.1002/pros.21383. Epub 2011 Mar 22. Prostate. 2011. PMID: 21432867 Free PMC article.
-
Neuroendocrine differentiation in usual-type prostatic adenocarcinoma: Molecular characterization and clinical significance.Prostate. 2020 Sep;80(12):1012-1023. doi: 10.1002/pros.24035. Epub 2020 Jul 10. Prostate. 2020. PMID: 32649013 Free PMC article.
-
Neuroendocrine pathogenesis in adenocarcinoma of the prostate.Ann Oncol. 2001;12 Suppl 2:S145-52. doi: 10.1093/annonc/12.suppl_2.s145. Ann Oncol. 2001. PMID: 11762343 Review.
Cited by
-
A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells.Oncotarget. 2015 Apr 10;6(10):7686-700. doi: 10.18632/oncotarget.3480. Oncotarget. 2015. PMID: 25797256 Free PMC article.
-
Neuroendocrine cells of prostate cancer: biologic functions and molecular mechanisms.Asian J Androl. 2019 May-Jun;21(3):291-295. doi: 10.4103/aja.aja_128_18. Asian J Androl. 2019. PMID: 30924452 Free PMC article.
-
MicroRNA-652 induces NED in LNCaP and EMT in PC3 prostate cancer cells.Oncotarget. 2018 Apr 10;9(27):19159-19176. doi: 10.18632/oncotarget.24937. eCollection 2018 Apr 10. Oncotarget. 2018. PMID: 29721191 Free PMC article.
-
Small-Cell Carcinoma of the Prostate - Challenges of Diagnosis and Treatment: A Next of Kin and Physician Perspective Piece.Oncol Ther. 2023 Sep;11(3):291-301. doi: 10.1007/s40487-023-00238-3. Epub 2023 Jun 25. Oncol Ther. 2023. PMID: 37358792 Free PMC article.
-
Targeting the adaptive molecular landscape of castration-resistant prostate cancer.EMBO Mol Med. 2015 Jul;7(7):878-94. doi: 10.15252/emmm.201303701. EMBO Mol Med. 2015. PMID: 25896606 Free PMC article. Review.
References
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. - PubMed
-
- Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Annals of Oncology. 2001;12(Suppl 2):S141–S144. - PubMed
-
- Brown JR, Wieczorek TJ, Shaffer K, Salgia R. Small-cell cancers, and an unusual reaction to chemotherapy. Case 1. Extrapulmonary small-cell carcinoma arising in the prostate. Journal of Clinical Oncology. 2003;21:2437–2438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

