Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial
- PMID: 22385038
- PMCID: PMC7161860
- DOI: 10.1111/j.1365-2133.2012.10923.x
Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial
Abstract
Background: The development of antibiotic resistance by microorganisms is an increasing problem in medicine. In chronic wounds, bacterial colonization is associated with impaired healing. Cold atmospheric plasma is an innovative promising tool to deal with these problems.
Objectives: The 5-min argon plasma treatment has already demonstrated efficacy in reducing bacterial numbers in chronic infected wounds in vivo. In this study we investigated a 2-min plasma treatment with the same device and the next-generation device, to assess safety and reduction in bacterial load, regardless of the kind of bacteria and their resistance level in chronic wounds.
Methods: Twenty-four patients with chronic infected wounds were treated in a prospective randomized controlled phase II study with 2 min of cold atmospheric argon plasma every day: 14 with MicroPlaSter alpha device, 10 with MicroPlaSter beta device (next-generation device) in addition to standard wound care. The patient acted as his/her own control. Bacterial species were detected by standard bacterial swabs and bacterial load by semiquantitative count on nitrocellulose filters. The plasma settings were the same as in the previous phase II study in which wounds were exposed for 5 min to argon plasma.
Results: Analysis of 70 treatments in 14 patients with the MicroPlaSter alpha device revealed a significant (40%, P<0.016) reduction in bacterial load in plasma-treated wounds, regardless of the species of bacteria. Analysis of 137 treatments in 10 patients with the MicroPlaSter beta device showed a highly significant reduction (23.5%, P<0.008) in bacterial load. No side-effects occurred and the treatment was well tolerated.
Conclusions: A 2-min treatment with either of two cold atmospheric argon plasma devices is a safe, painless and effective technique to decrease the bacterial load in chronic wounds.
© 2012 The Authors. BJD © 2012 British Association of Dermatologists.
Figures

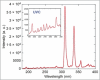


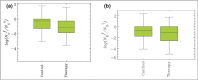
 , where
, where  . is the number of colonies after (before) treatment, respectively. (b) MicroPlaSter beta group with median log reduction of −1·1 in treated area vs. a −0·69 reduction in control area (P < 0·002).
. is the number of colonies after (before) treatment, respectively. (b) MicroPlaSter beta group with median log reduction of −1·1 in treated area vs. a −0·69 reduction in control area (P < 0·002).Similar articles
-
Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study.J Wound Care. 2015 May;24(5):196, 198-200, 202-3. doi: 10.12968/jowc.2015.24.5.196. J Wound Care. 2015. PMID: 25970756 Clinical Trial.
-
A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients.Br J Dermatol. 2010 Jul;163(1):78-82. doi: 10.1111/j.1365-2133.2010.09744.x. Epub 2010 Mar 5. Br J Dermatol. 2010. PMID: 20222930 Clinical Trial.
-
Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites.Wound Repair Regen. 2013 Nov-Dec;21(6):800-7. doi: 10.1111/wrr.12078. Epub 2013 Aug 12. Wound Repair Regen. 2013. PMID: 23937657 Clinical Trial.
-
[Cold atmospheric pressure plasma for the treatment of acute and chronic wounds].Hautarzt. 2020 Nov;71(11):855-862. doi: 10.1007/s00105-020-04696-y. Hautarzt. 2020. PMID: 32997219 Review. German.
-
Efficacy of Cold Atmospheric Plasma Therapy on Chronic Wounds: An Updated Systematic Review and Meta-Analysis of RCTs.Comput Math Methods Med. 2022 Oct 10;2022:5798857. doi: 10.1155/2022/5798857. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Nov 29;2023:9814529. doi: 10.1155/2023/9814529 PMID: 36262869 Free PMC article. Retracted. Review.
Cited by
-
Argon Plasma Exposure Augments Costimulatory Ligands and Cytokine Release in Human Monocyte-Derived Dendritic Cells.Int J Mol Sci. 2021 Apr 6;22(7):3790. doi: 10.3390/ijms22073790. Int J Mol Sci. 2021. PMID: 33917526 Free PMC article.
-
Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge.Sci Rep. 2018 Nov 12;8(1):16674. doi: 10.1038/s41598-018-35166-0. Sci Rep. 2018. PMID: 30420780 Free PMC article.
-
Advancing antimicrobial strategies for managing oral biofilm infections.Int J Oral Sci. 2019 Oct 1;11(3):28. doi: 10.1038/s41368-019-0062-1. Int J Oral Sci. 2019. PMID: 31570700 Free PMC article. Review.
-
Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis.Int Wound J. 2019 Feb;16(1):103-111. doi: 10.1111/iwj.12999. Epub 2018 Oct 12. Int Wound J. 2019. PMID: 30311743 Free PMC article.
-
Cold Atmospheric Plasma: A Promising Complementary Therapy for Squamous Head and Neck Cancer.PLoS One. 2015 Nov 20;10(11):e0141827. doi: 10.1371/journal.pone.0141827. eCollection 2015. PLoS One. 2015. PMID: 26588072 Free PMC article.
References
-
- Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost 2002; 28 (Suppl. 2):3–13. - PubMed
-
- Kurz X, Kahn SR, Abenhaim L et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence‐based report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. Int Angiol 1999; 18:83–102. - PubMed
-
- Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol 2007; 25:121–30. - PubMed
-
- Breidenbach WC, Trager S. Quantitative culture technique and infection in complex wounds of the extremities closed with free flaps. Plast Reconstr Surg 1995; 95:860–5. - PubMed
-
- Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997; 77:637–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

