A pilot study of IL-2Rα blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma
- PMID: 22383993
- PMCID: PMC3288003
- DOI: 10.1371/journal.pone.0031046
A pilot study of IL-2Rα blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma
Abstract
Background: Preclinical studies in mice have demonstrated that the prophylactic depletion of immunosuppressive regulatory T-cells (T(Regs)) through targeting the high affinity interleukin-2 (IL-2) receptor (IL-2Rα/CD25) can enhance anti-tumor immunotherapy. However, therapeutic approaches are complicated by the inadvertent inhibition of IL-2Rα expressing anti-tumor effector T-cells.
Objective: To determine if changes in the cytokine milieu during lymphopenia may engender differential signaling requirements that would enable unarmed anti-IL-2Rα monoclonal antibody (MAbs) to selectively deplete T(Regs) while permitting vaccine-stimulated immune responses.
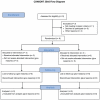
Methodology: A randomized placebo-controlled pilot study was undertaken to examine the ability of the anti-IL-2Rα MAb daclizumab, given at the time of epidermal growth factor receptor variant III (EGFRvIII) targeted peptide vaccination, to safely and selectively deplete T(Regs) in patients with glioblastoma (GBM) treated with lymphodepleting temozolomide (TMZ).
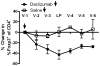
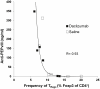
Results and conclusions: Daclizumab treatment (n = 3) was well-tolerated with no symptoms of autoimmune toxicity and resulted in a significant reduction in the frequency of circulating CD4+Foxp3+ TRegs in comparison to saline controls (n = 3)( p = 0.0464). A significant (p<0.0001) inverse correlation between the frequency of TRegs and the level of EGFRvIII specific humoral responses suggests the depletion of TRegs may be linked to increased vaccine-stimulated humoral immunity. These data suggest this approach deserves further study.
Trial registration: ClinicalTrials.gov NCT00626015.
Conflict of interest statement
Figures
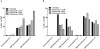
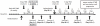
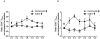
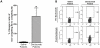
Similar articles
-
Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans.Blood. 2011 Sep 15;118(11):3003-12. doi: 10.1182/blood-2011-02-334565. Epub 2011 Jul 18. Blood. 2011. PMID: 21768296 Free PMC article. Clinical Trial.
-
CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients.Sci Transl Med. 2012 May 16;4(134):134ra62. doi: 10.1126/scitranslmed.3003330. Sci Transl Med. 2012. PMID: 22593175 Free PMC article. Clinical Trial.
-
CD25 Blockade Delays Regulatory T Cell Reconstitution and Does Not Prevent Graft-versus-Host Disease After Allogeneic Hematopoietic Cell Transplantation.Biol Blood Marrow Transplant. 2017 Mar;23(3):405-411. doi: 10.1016/j.bbmt.2016.12.624. Epub 2016 Dec 19. Biol Blood Marrow Transplant. 2017. PMID: 28007665 Free PMC article. Clinical Trial.
-
Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells.Ann N Y Acad Sci. 2009 Sep;1174:99-106. doi: 10.1111/j.1749-6632.2009.04939.x. Ann N Y Acad Sci. 2009. PMID: 19769742 Review.
-
Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis.Nat Rev Neurol. 2013 Jul;9(7):394-404. doi: 10.1038/nrneurol.2013.95. Epub 2013 Jun 4. Nat Rev Neurol. 2013. PMID: 23732529 Review.
Cited by
-
Biomarkers for glioma immunotherapy: the next generation.J Neurooncol. 2015 Jul;123(3):359-72. doi: 10.1007/s11060-015-1746-9. Epub 2015 Feb 28. J Neurooncol. 2015. PMID: 25724916 Free PMC article. Review.
-
Harnessing cytokines and chemokines for cancer therapy.Nat Rev Clin Oncol. 2022 Apr;19(4):237-253. doi: 10.1038/s41571-021-00588-9. Epub 2022 Jan 7. Nat Rev Clin Oncol. 2022. PMID: 34997230 Review.
-
Phenotypic and Functional Properties of Tumor-Infiltrating Regulatory T Cells.Mediators Inflamm. 2017;2017:5458178. doi: 10.1155/2017/5458178. Epub 2017 Dec 31. Mediators Inflamm. 2017. PMID: 29463952 Free PMC article. Review.
-
Immune-checkpoint blockade and active immunotherapy for glioma.Cancers (Basel). 2013 Nov 1;5(4):1379-412. doi: 10.3390/cancers5041379. Cancers (Basel). 2013. PMID: 24202450 Free PMC article.
-
Antibody-based immunotherapy for malignant glioma.Semin Oncol. 2014 Aug;41(4):496-510. doi: 10.1053/j.seminoncol.2014.06.004. Epub 2014 Jun 12. Semin Oncol. 2014. PMID: 25173142 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P50 CA108786/CA/NCI NIH HHS/United States
- P50 NS020023/NS/NINDS NIH HHS/United States
- R01 CA097222/CA/NCI NIH HHS/United States
- R01-CA097222/CA/NCI NIH HHS/United States
- UL1 RR024128/RR/NCRR NIH HHS/United States
- P50-CA108786/CA/NCI NIH HHS/United States
- R21 NS067980/NS/NINDS NIH HHS/United States
- R21 CA132891/CA/NCI NIH HHS/United States
- 1UL2-RR024128/RR/NCRR NIH HHS/United States
- P50-NS020023/NS/NINDS NIH HHS/United States
- R21-CA132891/CA/NCI NIH HHS/United States
- R21-NS067980/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

