Retroviral cyclin enhances cyclin-dependent kinase-8 activity
- PMID: 22379099
- PMCID: PMC3347271
- DOI: 10.1128/JVI.07006-11
Retroviral cyclin enhances cyclin-dependent kinase-8 activity
Abstract
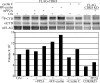
Alterations in the functional levels of cyclin-dependent kinase-8 (CDK8) or its partner, cyclin C, have been clearly associated with cancers, including colon cancer, melanoma, and osteosarcoma. Walleye dermal sarcoma virus encodes a retroviral cyclin (RV-cyclin) that localizes to interchromatin granule clusters and binds CDK8. It also binds to the Aα subunit (PR65) of protein phosphatase 2A (PP2A). Binding to the Aα subunit excludes the regulatory B subunit, but not the catalytic C subunit, in a manner similar to that of T antigens of the small DNA tumor viruses. The expression of the RV-cyclin enhances the activity of immune affinity-purified CDK8 in vitro for RNA polymerase II carboxy-terminal domain (CTD) and histone H3 substrates. PP2A also enhances CDK8 kinase activity in vitro for the CTD but not for histone H3. The PP2A enhancement of CDK8 is independent of RV-cyclin expression and likely plays a role in the normal regulation of CDK8. The manipulation of endogenous PP2A activity by inhibition, amendment, or depletion confirmed its role in CDK8 activation by triggering CDK8 autophosphorylation. Although RV-cyclin and PP2A both enhance CDK8 activity, their actions are uncoupled and additive in kinase reactions. PP2A may be recruited to CDK8 in the Mediator complex by a specific PP2A B subunit or additionally by the RV-cyclin in infected cells, but the RV-cyclin appears to activate CDK8 directly and in a manner independent of its physical association with PP2A.
Figures


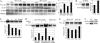
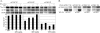



Similar articles
-
Retroviral cyclin controls cyclin-dependent kinase 8-mediated transcription elongation and reinitiation.J Virol. 2015 May;89(10):5450-61. doi: 10.1128/JVI.00464-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741012 Free PMC article.
-
The retroviral cyclin of walleye dermal sarcoma virus binds cyclin-dependent kinases 3 and 8.Virology. 2011 Jan 20;409(2):299-307. doi: 10.1016/j.virol.2010.10.022. Epub 2010 Nov 9. Virology. 2011. PMID: 21067790 Free PMC article.
-
Walleye dermal sarcoma virus cyclin interacts with components of the mediator complex and the RNA polymerase II holoenzyme.J Virol. 2002 Aug;76(16):8031-9. doi: 10.1128/jvi.76.16.8031-8039.2002. J Virol. 2002. PMID: 12134008 Free PMC article.
-
Dysregulation of CDK8 and Cyclin C in tumorigenesis.J Genet Genomics. 2011 Oct 20;38(10):439-52. doi: 10.1016/j.jgg.2011.09.002. Epub 2011 Sep 16. J Genet Genomics. 2011. PMID: 22035865 Free PMC article. Review.
-
Mediator kinase module and human tumorigenesis.Crit Rev Biochem Mol Biol. 2015;50(5):393-426. doi: 10.3109/10409238.2015.1064854. Epub 2015 Jul 16. Crit Rev Biochem Mol Biol. 2015. PMID: 26182352 Free PMC article. Review.
Cited by
-
Exploitation of the Mediator complex by viruses.PLoS Pathog. 2022 Apr 21;18(4):e1010422. doi: 10.1371/journal.ppat.1010422. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35446926 Free PMC article. No abstract available.
-
Beyond the coding genome: non-coding mutations and cancer.Front Biosci (Landmark Ed). 2020 Jun 1;25(10):1828-1838. doi: 10.2741/4879. Front Biosci (Landmark Ed). 2020. PMID: 32472759 Free PMC article. Review.
-
Tumor-suppressive effects of CDK8 in endometrial cancer cells.Cell Cycle. 2013 Mar 15;12(6):987-99. doi: 10.4161/cc.24003. Epub 2013 Mar 1. Cell Cycle. 2013. PMID: 23454913 Free PMC article.
-
Cyclin-Dependent Kinases 8 and 19 Regulate Host Cell Metabolism during Dengue Virus Serotype 2 Infection.Viruses. 2020 Jun 17;12(6):654. doi: 10.3390/v12060654. Viruses. 2020. PMID: 32560467 Free PMC article.
-
Retroviral cyclin controls cyclin-dependent kinase 8-mediated transcription elongation and reinitiation.J Virol. 2015 May;89(10):5450-61. doi: 10.1128/JVI.00464-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741012 Free PMC article.
References
-
- Akoulitchev S, Chuikov S, Reinberg D. 2000. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407:102–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

