Evidence for a stepwise program of extrathymic T cell development within the human tonsil
- PMID: 22378041
- PMCID: PMC3314444
- DOI: 10.1172/JCI46125
Evidence for a stepwise program of extrathymic T cell development within the human tonsil
Abstract
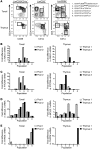
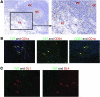
The development of a broad repertoire of T cells, which is essential for effective immune function, occurs in the thymus. Although some data suggest that T cell development can occur extrathymically, many researchers remain skeptical that extrathymic T cell development has an important role in generating the T cell repertoire in healthy individuals. However, it may be important in the setting of poor thymic function or congenital deficit and in the context of autoimmunity, cancer, or regenerative medicine. Here, we report evidence that a stepwise program of T cell development occurs within the human tonsil. We identified 5 tonsillar T cell developmental intermediates: (a) CD34⁺CD38dimLin⁻ cells, which resemble multipotent progenitors in the bone marrow and thymus; (b) more mature CD34⁺CD38brightLin⁻ cells; (c) CD34⁺CD1a⁺CD11c⁻ cells, which resemble committed T cell lineage precursors in the thymus; (d) CD34⁻CD1a⁺CD3⁻CD11c⁻ cells, which resemble CD4⁺CD8⁺ double-positive T cells in the thymus; and (e) CD34⁻CD1a⁺CD3⁺CD11c⁻ cells. The phenotype of each subset closely resembled that of its thymic counterpart. The last 4 populations expressed RAG1 and PTCRA, genes required for TCR rearrangement, and all 5 subsets were capable of ex vivo T cell differentiation. TdT⁺ cells found within the tonsillar fibrous scaffold expressed CD34 and/or CD1a, indicating that this distinct anatomic region contributes to pre-T cell development, as does the subcapsular region of the thymus. Thus, we provide evidence of a role for the human tonsil in a comprehensive program of extrathymic T cell development.
Figures






Comment in
-
T cell development: Tonsils turn out T cells too.Nat Rev Immunol. 2012 Mar 9;12(4):232. doi: 10.1038/nri3196. Nat Rev Immunol. 2012. PMID: 22402672 No abstract available.
Similar articles
-
CD34+CD38dim cells in the human thymus can differentiate into T, natural killer, and dendritic cells but are distinct from pluripotent stem cells.Blood. 1996 Jun 15;87(12):5196-206. Blood. 1996. PMID: 8652833
-
Double-positive thymocytes select mucosal-associated invariant T cells.J Immunol. 2013 Dec 15;191(12):6002-9. doi: 10.4049/jimmunol.1301212. Epub 2013 Nov 15. J Immunol. 2013. PMID: 24244014
-
Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells.Blood. 2009 Jul 30;114(5):972-82. doi: 10.1182/blood-2008-10-187013. Epub 2009 Jun 2. Blood. 2009. PMID: 19491395
-
Development and functional properties of thymic and extrathymic T lymphocytes.Crit Rev Immunol. 2008;28(5):441-66. doi: 10.1615/critrevimmunol.v28.i5.40. Crit Rev Immunol. 2008. PMID: 19166388 Review.
-
Thymic differentiation of TCR alpha beta(+) CD8 alpha alpha(+) IELs.Immunol Rev. 2007 Feb;215:178-88. doi: 10.1111/j.1600-065X.2006.00488.x. Immunol Rev. 2007. PMID: 17291288 Review.
Cited by
-
Making T cells functional: a new solution for the athymic BMT recipients.Acta Pharmacol Sin. 2013 Feb;34(2):189-90. doi: 10.1038/aps.2012.193. Acta Pharmacol Sin. 2013. PMID: 23381109 Free PMC article. No abstract available.
-
Genetic T-cell receptor diversity at 1 year following allogeneic hematopoietic stem cell transplantation.Leukemia. 2020 May;34(5):1422-1432. doi: 10.1038/s41375-019-0654-y. Epub 2019 Nov 26. Leukemia. 2020. PMID: 31772297
-
The intestinal γδ T cells: functions in the gut and in the distant organs.Front Immunol. 2023 Jun 16;14:1206299. doi: 10.3389/fimmu.2023.1206299. eCollection 2023. Front Immunol. 2023. PMID: 37398661 Free PMC article. Review.
-
Human Peripheral CD4(+) Vδ1(+) γδT Cells Can Develop into αβT Cells.Front Immunol. 2014 Dec 17;5:645. doi: 10.3389/fimmu.2014.00645. eCollection 2014. Front Immunol. 2014. PMID: 25709606 Free PMC article.
-
Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion.Curr Top Microbiol Immunol. 2019;421:229-265. doi: 10.1007/978-3-030-15138-6_10. Curr Top Microbiol Immunol. 2019. PMID: 31123892 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

