Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia
- PMID: 22368275
- PMCID: PMC3482472
- DOI: 10.4049/jimmunol.1102272
Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia
Abstract
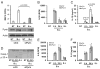
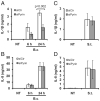
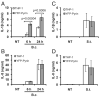
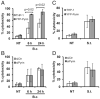
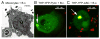
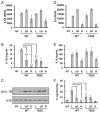
Burkholderia cenocepacia is an opportunistic pathogen that causes chronic infection and induces progressive respiratory inflammation in cystic fibrosis patients. Recognition of bacteria by mononuclear cells generally results in the activation of caspase-1 and processing of IL-1β, a major proinflammatory cytokine. In this study, we report that human pyrin is required to detect intracellular B. cenocepacia leading to IL-1β processing and release. This inflammatory response involves the host adapter molecule ASC and the bacterial type VI secretion system (T6SS). Human monocytes and THP-1 cells stably expressing either small interfering RNA against pyrin or YFP-pyrin and ASC (YFP-ASC) were infected with B. cenocepacia and analyzed for inflammasome activation. B. cenocepacia efficiently activates the inflammasome and IL-1β release in monocytes and THP-1. Suppression of pyrin levels in monocytes and THP-1 cells reduced caspase-1 activation and IL-1β release in response to B. cenocepacia challenge. In contrast, overexpression of pyrin or ASC induced a robust IL-1β response to B. cenocepacia, which correlated with enhanced host cell death. Inflammasome activation was significantly reduced in cells infected with T6SS-defective mutants of B. cenocepacia, suggesting that the inflammatory reaction is likely induced by an as yet uncharacterized effector(s) of the T6SS. Together, we show for the first time, to our knowledge, that in human mononuclear cells infected with B. cenocepacia, pyrin associates with caspase-1 and ASC forming an inflammasome that upregulates mononuclear cell IL-1β processing and release.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


Similar articles
-
A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation.Cell Host Microbe. 2016 May 11;19(5):664-74. doi: 10.1016/j.chom.2016.04.004. Epub 2016 Apr 28. Cell Host Microbe. 2016. PMID: 27133449
-
Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages.PLoS One. 2012;7(7):e41726. doi: 10.1371/journal.pone.0041726. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848580 Free PMC article.
-
Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways.J Virol. 2011 Dec;85(23):12505-17. doi: 10.1128/JVI.00410-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957307 Free PMC article.
-
["The inflammasomes"].Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(5):346-54. doi: 10.2177/jsci.34.346. Nihon Rinsho Meneki Gakkai Kaishi. 2011. PMID: 22041421 Review. Japanese.
-
Inflammasomes and Fibrosis.Front Immunol. 2021 Jun 11;12:643149. doi: 10.3389/fimmu.2021.643149. eCollection 2021. Front Immunol. 2021. PMID: 34177893 Free PMC article. Review.
Cited by
-
RIPK3 Promotes Mefv Expression and Pyrin Inflammasome Activation via Modulation of mTOR Signaling.J Immunol. 2020 Nov 15;205(10):2778-2785. doi: 10.4049/jimmunol.2000244. Epub 2020 Sep 28. J Immunol. 2020. PMID: 32989095 Free PMC article.
-
The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome.Cell Host Microbe. 2016 Sep 14;20(3):296-306. doi: 10.1016/j.chom.2016.07.018. Epub 2016 Aug 25. Cell Host Microbe. 2016. PMID: 27569559 Free PMC article.
-
Bacterial type VI secretion system (T6SS): an evolved molecular weapon with diverse functionality.Biotechnol Lett. 2023 Mar;45(3):309-331. doi: 10.1007/s10529-023-03354-2. Epub 2023 Jan 23. Biotechnol Lett. 2023. PMID: 36683130 Review.
-
Live and inactivated Salmonella enterica serovar Typhimurium stimulate similar but distinct transcriptome profiles in bovine macrophages and dendritic cells.Vet Res. 2016 Mar 22;47:46. doi: 10.1186/s13567-016-0328-y. Vet Res. 2016. PMID: 27000047 Free PMC article.
-
The PRY/SPRY domain of pyrin/TRIM20 interacts with β2-microglobulin to promote inflammasome formation.Sci Rep. 2021 Dec 8;11(1):23613. doi: 10.1038/s41598-021-03073-6. Sci Rep. 2021. PMID: 34880353 Free PMC article.
References
-
- Magni A, Giordano A, Mancini C, Pecoraro C, Varesi P, Quattrucci S, Trancassini M. Emerging cystic fibrosis pathogens: incidence and antimicrobial resistance. New Microbiol. 2007;30:59–62. - PubMed
-
- Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. - PubMed
-
- Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest. 2009;136:1554–1560. - PubMed
-
- Desjardins M, Celis JE, van Meer G, Dieplinger H, Jahraus A, Griffiths G, Huber LA. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194–32200. - PubMed
-
- Desjardins M, Nzala NN, Corsini R, Rondeau C. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J Cell Sci. 1997;110:2303–2314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL076278/HL/NHLBI NIH HHS/United States
- HL76278/HL/NHLBI NIH HHS/United States
- R01 HL094586-01A1/HL/NHLBI NIH HHS/United States
- R21 HL102724/HL/NHLBI NIH HHS/United States
- HL089440/HL/NHLBI NIH HHS/United States
- R21 AI083871/AI/NIAID NIH HHS/United States
- R01 HL089440/HL/NHLBI NIH HHS/United States
- HL102724/HL/NHLBI NIH HHS/United States
- R21 AI083871-01/AI/NIAID NIH HHS/United States
- R01 HL094586-03/HL/NHLBI NIH HHS/United States
- R01 HL094586-02/HL/NHLBI NIH HHS/United States
- AI083871/AI/NIAID NIH HHS/United States
- HL094586/HL/NHLBI NIH HHS/United States
- R01 HL094586/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

