VEGF signaling inside vascular endothelial cells and beyond
- PMID: 22366328
- PMCID: PMC4030755
- DOI: 10.1016/j.ceb.2012.02.002
VEGF signaling inside vascular endothelial cells and beyond
Abstract
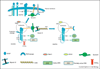
Vascular endothelial growth factor-A (VEGF-A) has long been recognized as the key regulator of vascular development and function in health and disease. VEGF is a secreted polypeptide that binds to transmembrane tyrosine kinase VEGF receptors on the plasma membrane, inducing their dimerization, activation and assembly of a membrane-proximal signaling complex. Recent studies have revealed that many key events of VEGFR signaling occur inside the endothelial cell and are regulated by endosomal receptor trafficking. Plasma membrane VEGFR interacting molecules, including vascular guidance receptors Neuropilins and Ephrins also regulate VEGFR endocytosis and trafficking. VEGF signaling is increasingly recognized for its roles outside of the vascular system, notably during neural development, and blood vessels regulate epithelial branching morphogenesis. We review here recent advances in our understanding of VEGF signaling and its biological roles.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
VEGF Receptor Tyrosine Kinases: Key Regulators of Vascular Function.Curr Top Dev Biol. 2017;123:433-482. doi: 10.1016/bs.ctdb.2016.10.001. Epub 2016 Nov 30. Curr Top Dev Biol. 2017. PMID: 28236974 Review.
-
Vascular endothelial growth factor induces branching morphogenesis/tubulogenesis in renal epithelial cells in a neuropilin-dependent fashion.Mol Cell Biol. 2005 Sep;25(17):7441-8. doi: 10.1128/MCB.25.17.7441-7448.2005. Mol Cell Biol. 2005. PMID: 16107693 Free PMC article.
-
VEGF-A121a binding to Neuropilins - A concept revisited.Cell Adh Migr. 2018 May 4;12(3):204-214. doi: 10.1080/19336918.2017.1372878. Epub 2017 Nov 2. Cell Adh Migr. 2018. PMID: 29095088 Free PMC article. Review.
-
Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis.Mol Med. 2006 Jul-Aug;12(7-8):127-36. doi: 10.2119/2006-00024.Man. Mol Med. 2006. PMID: 17088944 Free PMC article.
-
Selective inhibition of vascular endothelial growth factor receptor-2 (VEGFR-2) identifies a central role for VEGFR-2 in human aortic endothelial cell responses to VEGF.J Recept Signal Transduct Res. 2003;23(2-3):239-54. doi: 10.1081/rrs-120025567. J Recept Signal Transduct Res. 2003. PMID: 14626450
Cited by
-
Vessel Enlargement in Development and Pathophysiology.Front Physiol. 2021 Feb 25;12:639645. doi: 10.3389/fphys.2021.639645. eCollection 2021. Front Physiol. 2021. PMID: 33716786 Free PMC article. Review.
-
The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis.Dev Cell. 2013 Apr 29;25(2):156-68. doi: 10.1016/j.devcel.2013.03.019. Dev Cell. 2013. PMID: 23639442 Free PMC article.
-
The usefulness of lutein/trypan blue vital dye for the staining of corneal endothelium: a pilot study on DMEK pretreated tissues.Graefes Arch Clin Exp Ophthalmol. 2023 May;261(5):1321-1329. doi: 10.1007/s00417-022-05909-x. Epub 2022 Nov 29. Graefes Arch Clin Exp Ophthalmol. 2023. PMID: 36445446 Free PMC article.
-
Salvianolic acid B promotes angiogenesis and inhibits cardiomyocyte apoptosis by regulating autophagy in myocardial ischemia.Chin Med. 2023 Nov 28;18(1):155. doi: 10.1186/s13020-023-00859-w. Chin Med. 2023. PMID: 38017536 Free PMC article.
-
An inside view: VEGF receptor trafficking and signaling.Physiology (Bethesda). 2012 Aug;27(4):213-22. doi: 10.1152/physiol.00016.2012. Physiology (Bethesda). 2012. PMID: 22875452 Free PMC article. Review.
References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. - PubMed
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

