Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload
- PMID: 22362893
- PMCID: PMC3360147
- DOI: 10.1096/fj.11-190728
Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload
Abstract
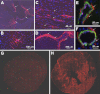
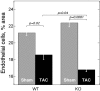
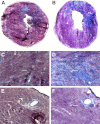
Thrombospondin-4 (TSP-4) expression increases dramatically in hypertrophic and failing hearts in rodent models and in humans. The aim of this study was to address the function of TSP-4 in the heart. TSP-4-knockout (Thbs4(-/-)) and wild-type (WT) mice were subjected to transverse aortic constriction (TAC) to increase left ventricle load. After 2 wk, Thbs4(-/-) mice had a significantly higher heart weight/body weight ratio than WT mice. The additional increase in the heart weight in TAC Thbs4(-/-) mice was due to increased deposition of extracellular matrix (ECM). The levels of interstitial collagens were higher in the knockout mice, but the size of cardiomyocytes and apoptosis in the myocardium was unaffected by TSP-4 deficiency, suggesting that increased reactive fibrosis was the primary cause of the higher heart weight. The increased ECM deposition in Thbs4(-/-) mice was accompanied by changes in functional parameters of the heart and decreased vessel density. The expression of inflammatory and fibrotic genes known to be influential in myocardial remodeling changed as a result of TSP-4 deficiency in vivo and as a result of incubation of cells with recombinant TSP-4 in vitro. Thus, TSP-4 is involved in regulating the adaptive responses of the heart to pressure overload, suggesting its important role in myocardial remodeling. Our study showed a direct influence of TSP-4 on heart function and to identify the mechanism of its effects on heart remodeling.
Figures


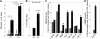

Similar articles
-
Thrombospondin-4 mediates cardiovascular remodelling in angiotensin II-induced hypertension.Cardiovasc Pathol. 2018 Jul-Aug;35:12-19. doi: 10.1016/j.carpath.2018.03.003. Epub 2018 Apr 7. Cardiovasc Pathol. 2018. PMID: 29729633
-
Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism.Hypertension. 2011 Nov;58(5):902-11. doi: 10.1161/HYPERTENSIONAHA.111.175323. Epub 2011 Sep 26. Hypertension. 2011. PMID: 21947471 Free PMC article.
-
Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload.Hypertension. 2006 Apr;47(4):711-7. doi: 10.1161/01.HYP.0000208840.30778.00. Epub 2006 Feb 27. Hypertension. 2006. PMID: 16505197
-
Thrombospondin-4, tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14: novel extracellular matrix modulating factors in cardiac remodelling.Ann Med. 2012 Dec;44(8):793-804. doi: 10.3109/07853890.2011.614635. Epub 2012 Mar 1. Ann Med. 2012. PMID: 22380695 Review.
-
The role of the thrombospondins in healing myocardial infarcts.Cardiovasc Hematol Agents Med Chem. 2007 Jan;5(1):21-7. doi: 10.2174/187152507779315813. Cardiovasc Hematol Agents Med Chem. 2007. PMID: 17266545 Review.
Cited by
-
Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload.Am J Physiol Heart Circ Physiol. 2012 Jul 15;303(2):H234-40. doi: 10.1152/ajpheart.00227.2012. Epub 2012 May 18. Am J Physiol Heart Circ Physiol. 2012. PMID: 22610170 Free PMC article.
-
De-obstruction of bladder outlet in humans reverses organ remodelling by normalizing the expression of key transcription factors.BMC Urol. 2024 Feb 7;24(1):33. doi: 10.1186/s12894-024-01417-8. BMC Urol. 2024. PMID: 38326801 Free PMC article.
-
The Role of Matrix Proteins in Cardiac Pathology.Int J Mol Sci. 2022 Jan 25;23(3):1338. doi: 10.3390/ijms23031338. Int J Mol Sci. 2022. PMID: 35163259 Free PMC article. Review.
-
Dissecting the cellular landscape and transcriptome network in viral myocarditis by single-cell RNA sequencing.iScience. 2022 Feb 2;25(3):103865. doi: 10.1016/j.isci.2022.103865. eCollection 2022 Mar 18. iScience. 2022. PMID: 35243228 Free PMC article.
-
The secretome as a biomarker and functional agent in heart failure.J Cardiovasc Aging. 2023 Jul;3(3):27. doi: 10.20517/jca.2023.15. Epub 2023 Jun 9. J Cardiovasc Aging. 2023. PMID: 37484982 Free PMC article.
References
-
- Adams J. C. (2001) Thrombospondins: multifunctional regulators of cell interactions. Annu. Rev. Cell Dev. Biol. 17, 25–51 - PubMed
-
- Stenina O. I., Desai S. Y., Krukovets I., Kight K., Janigro D., Topol E. J., Plow E. F. (2003) Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation 108, 1514–1519 - PubMed
-
- Mustonen E., Aro J., Puhakka J., Ilves M., Soini Y., Leskinen H., Ruskoaho H., Rysa J. (2008) Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem. Biophys. Res. Commun. 373, 186–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

