Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host
- PMID: 22360982
- PMCID: PMC3429131
- DOI: 10.1016/j.jamcollsurg.2011.12.034
Ex vivo interleukin-12-priming during CD8(+) T cell activation dramatically improves adoptive T cell transfer antitumor efficacy in a lymphodepleted host
Abstract
Background: Clinical application of adoptive T cell therapy has been hindered by an inability to generate adequate numbers of nontolerized, functionally active, tumor-specific T cells, which can persist in vivo. In order to address this, we evaluated the impact of interleukin (IL)-12 signaling during tumor-specific CD8(+) T cell priming in terms of persistence and antitumor efficacy using an established B16 melanoma tumor adoptive therapy model.
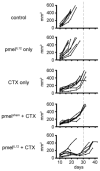
Study design: B6 mice were injected subcutaneously with B16 melanoma tumor cells. On day 12 of tumor growth, mice were preconditioned with cyclophosphamide (4mg dose, intraperitoneally), and 1 day later were treated by adoptive transfer of tumor-specific pmel-1 CD8(+) T cells primed ex vivo 3 days earlier with both IL-12 and antigen (hGP100(25-33) peptide) or antigen only. Tumors were measured biweekly, and infused donor T cells were analyzed for persistence, localization to the tumor, phenotype, and effector function.
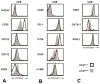
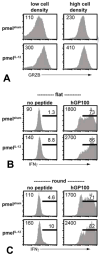
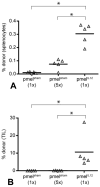
Results: Adoptive transfer of tumor-specific CD8(+) T cells primed with IL-12 was significantly more effective in reducing tumor burden in mice preconditioned with cyclophosphamide compared with transfer of T cells primed without IL-12. This enhanced antitumor response was associated with increased frequencies of infused T cells in the periphery and tumor as well as elevated expression of effector molecules including granzyme B and interferon-γ (IFNγ).
Conclusions: Our findings demonstrate that ex vivo priming of tumor-specific CD8(+) T cells with IL-12 dramatically improves their in vivo persistence and therapeutic ability on transfer to tumor-bearing mice. These findings can be directly applied as novel clinical trial strategies.
Copyright © 2012 American College of Surgeons. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Ex vivo culture with interleukin (IL)-12 improves CD8(+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin.Cancer Res. 2006 May 1;66(9):4913-21. doi: 10.1158/0008-5472.CAN-05-3507. Cancer Res. 2006. PMID: 16651448
-
Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells.Cancer Immunol Immunother. 2015 May;64(5):539-49. doi: 10.1007/s00262-015-1655-y. Epub 2015 Feb 13. Cancer Immunol Immunother. 2015. PMID: 25676709 Free PMC article.
-
Enhanced sensitivity to IL-2 signaling regulates the clinical responsiveness of IL-12-primed CD8(+) T cells in a melanoma model.J Immunol. 2011 May 1;186(9):5068-77. doi: 10.4049/jimmunol.1003317. Epub 2011 Mar 23. J Immunol. 2011. PMID: 21430221 Free PMC article.
-
Adoptive T-cell transfer in melanoma.Immunotherapy. 2013 Jan;5(1):79-90. doi: 10.2217/imt.12.143. Immunotherapy. 2013. PMID: 23256800 Review.
-
Brief in vitro IL-12 conditioning of CD8 + T Cells for anticancer adoptive T cell therapy.Cancer Immunol Immunother. 2021 Oct;70(10):2751-2759. doi: 10.1007/s00262-021-02887-7. Epub 2021 May 8. Cancer Immunol Immunother. 2021. PMID: 33966093 Free PMC article. Review.
Cited by
-
IL-2Rα mediates temporal regulation of IL-2 signaling and enhances immunotherapy.Sci Transl Med. 2015 Oct 28;7(311):311ra170. doi: 10.1126/scitranslmed.aac8155. Sci Transl Med. 2015. PMID: 26511507 Free PMC article.
-
Trial Watch: Adoptive cell transfer for anticancer immunotherapy.Oncoimmunology. 2013 May 1;2(5):e24238. doi: 10.4161/onci.24238. Oncoimmunology. 2013. PMID: 23762803 Free PMC article.
-
Adoptive T Cell Therapy with IL-12-Preconditioned Low-Avidity T Cells Prevents Exhaustion and Results in Enhanced T Cell Activation, Enhanced Tumor Clearance, and Decreased Risk for Autoimmunity.J Immunol. 2020 Sep 1;205(5):1449-1460. doi: 10.4049/jimmunol.2000007. Epub 2020 Jul 31. J Immunol. 2020. PMID: 32737148 Free PMC article.
-
IFN-γ: A cytokine at the right time, is in the right place.Semin Immunol. 2019 Jun;43:101280. doi: 10.1016/j.smim.2019.05.002. Epub 2019 Jun 17. Semin Immunol. 2019. PMID: 31221552 Free PMC article. Review.
-
Membrane-organizing protein moesin controls Treg differentiation and antitumor immunity via TGF-β signaling.J Clin Invest. 2017 Apr 3;127(4):1321-1337. doi: 10.1172/JCI89281. Epub 2017 Mar 13. J Clin Invest. 2017. PMID: 28287407 Free PMC article.
References
-
- Rosenberg SA. Karnofsky Memorial Lecture. The immunotherapy and gene therapy of cancer. J Clin Oncol. 1992;10:180–99. - PubMed
-
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. - PubMed
-
- Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

