Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib
- PMID: 22356324
- PMCID: PMC3724515
- DOI: 10.1056/NEJMoa1112302
Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib
Abstract
Background: Approximately 50% of melanomas harbor activating (V600) mutations in the serine-threonine protein kinase B-RAF (BRAF). The oral BRAF inhibitor vemurafenib (PLX4032) frequently produced tumor regressions in patients with BRAF V600-mutant metastatic melanoma in a phase 1 trial and improved overall survival in a phase 3 trial.
Methods: We designed a multicenter phase 2 trial of vemurafenib in patients with previously treated BRAF V600-mutant metastatic melanoma to investigate the efficacy of vemurafenib with respect to overall response rate (percentage of treated patients with a tumor response), duration of response, and overall survival. The primary end point was the overall response rate as ascertained by the independent review committee; overall survival was a secondary end point.
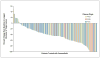
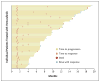
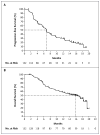
Results: A total of 132 patients had a median follow-up of 12.9 months (range, 0.6 to 20.1). The confirmed overall response rate was 53% (95% confidence interval [CI], 44 to 62; 6% with a complete response and 47% with a partial response), the median duration of response was 6.7 months (95% CI, 5.6 to 8.6), and the median progression-free survival was 6.8 months (95% CI, 5.6 to 8.1). Primary progression was observed in only 14% of patients. Some patients had a response after receiving vemurafenib for more than 6 months. The median overall survival was 15.9 months (95% CI, 11.6 to 18.3). The most common adverse events were grade 1 or 2 arthralgia, rash, photosensitivity, fatigue, and alopecia. Cutaneous squamous-cell carcinomas (the majority, keratoacanthoma type) were diagnosed in 26% of patients.
Conclusions: Vemurafenib induces clinical responses in more than half of patients with previously treated BRAF V600-mutant metastatic melanoma. In this study with a long follow-up, the median overall survival was approximately 16 months. (Funded by Hoffmann-La Roche; ClinicalTrials.gov number, NCT00949702.).
Figures
Similar articles
-
Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study.Lancet Oncol. 2014 Apr;15(4):436-44. doi: 10.1016/S1470-2045(14)70051-8. Epub 2014 Feb 27. Lancet Oncol. 2014. PMID: 24582505 Clinical Trial.
-
Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study.Lancet Oncol. 2014 Aug;15(9):954-65. doi: 10.1016/S1470-2045(14)70301-8. Epub 2014 Jul 15. Lancet Oncol. 2014. PMID: 25037139 Clinical Trial.
-
Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study.Lancet Oncol. 2014 Mar;15(3):323-32. doi: 10.1016/S1470-2045(14)70012-9. Epub 2014 Feb 7. Lancet Oncol. 2014. PMID: 24508103 Free PMC article. Clinical Trial.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
-
Drug safety evaluation of vemurafenib in the treatment of melanoma.Expert Opin Drug Saf. 2013 Sep;12(5):767-75. doi: 10.1517/14740338.2013.813017. Epub 2013 Jun 26. Expert Opin Drug Saf. 2013. PMID: 23800008 Review.
Cited by
-
Contribution of Beta-HPV Infection and UV Damage to Rapid-Onset Cutaneous Squamous Cell Carcinoma during BRAF-Inhibition Therapy.Clin Cancer Res. 2015 Jun 1;21(11):2624-34. doi: 10.1158/1078-0432.CCR-14-2667. Epub 2015 Feb 27. Clin Cancer Res. 2015. PMID: 25724524 Free PMC article.
-
Towards precision medicine.Nat Rev Genet. 2016 Aug 16;17(9):507-22. doi: 10.1038/nrg.2016.86. Nat Rev Genet. 2016. PMID: 27528417 Review.
-
Targeting p53 for Melanoma Treatment: Counteracting Tumour Proliferation, Dissemination and Therapeutic Resistance.Cancers (Basel). 2021 Apr 1;13(7):1648. doi: 10.3390/cancers13071648. Cancers (Basel). 2021. PMID: 33916029 Free PMC article.
-
C-RAF mutations confer resistance to RAF inhibitors.Cancer Res. 2013 Aug 1;73(15):4840-51. doi: 10.1158/0008-5472.CAN-12-4089. Epub 2013 Jun 4. Cancer Res. 2013. PMID: 23737487 Free PMC article.
-
The next steps in next-gen sequencing of cancer genomes.J Clin Invest. 2015 Feb;125(2):462-8. doi: 10.1172/JCI68339. Epub 2015 Feb 2. J Clin Invest. 2015. PMID: 25642706 Free PMC article. Review.
References
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. [Erratum, N Engl J Med 2004;351:2461.] - PubMed
-
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–51. - PubMed
-
- Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–54. - PMC - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. [Erratum, J Clin Oncol 2000; 18:2351.] - PubMed
-
- Falkson CI, Ibrahim J, Kirkwood JM, Coates AS, Atkins MB, Blum RH. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:1743–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
