Mechanisms regulating the secretion of the promalignancy chemokine CCL5 by breast tumor cells: CCL5's 40s loop and intracellular glycosaminoglycans
- PMID: 22355269
- PMCID: PMC3281937
- DOI: 10.1593/neo.111122
Mechanisms regulating the secretion of the promalignancy chemokine CCL5 by breast tumor cells: CCL5's 40s loop and intracellular glycosaminoglycans
Abstract
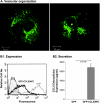
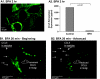
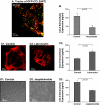
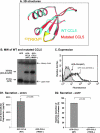
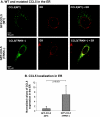
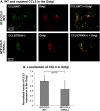
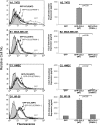
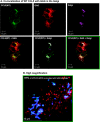
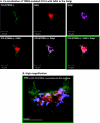
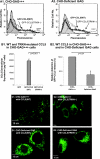
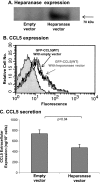
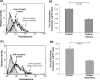
The chemokine CCL5 (RANTES) plays active promalignancy roles in breast malignancy. The secretion of CCL5 by breast tumor cells is an important step in its tumor-promoting activities; therefore, inhibition of CCL5 secretion may have antitumorigenic effects. We demonstrate that, in breast tumor cells, CCL5 secretion necessitated the trafficking of CCL5-containing vesicles on microtubules from the endoplasmic reticulum (ER) to the post-Golgi stage, and CCL5 release was regulated by the rigidity of the actin cytoskeleton. Focusing on the 40s loop of CCL5, we found that the (43)TRKN(46) sequence of CCL5 was indispensable for its inclusion in motile vesicles, and for its secretion. The TRKN-mutated chemokine reached the Golgi, but trafficked along the ER-to-post-Golgi route differently than the wild-type (WT) chemokine. Based on the studies showing that the 40s loop of CCL5 mediates its binding to glycosaminoglycans (GAG), we analyzed the roles of GAG in regulating CCL5 secretion. TRKN-mutated CCL5 had lower propensity for colocalization with GAG in the Golgi compared to the WT chemokine. Secretion of WT CCL5 was significantly reduced in CHO mutant cells deficient in GAG synthesis, and the WT chemokine acquired an ER-like distribution in these cells, similar to that of TRKN-mutated CCL5 in GAG-expressing cells. The release of WT CCL5 was also reduced after inhibition of GAG presence/synthesis by intracellular expression of heparanase, inhibition of GAG sulfation, and sulfate deprivation. The need for a (43)TRKN(46) motif and for a GAG-mediated process in CCL5 secretion may enable the future design of modalities that prevent CCL5 release by breast tumor cells.
Figures
Similar articles
-
Breast cancer: coordinated regulation of CCL2 secretion by intracellular glycosaminoglycans and chemokine motifs.Neoplasia. 2014 Sep;16(9):723-40. doi: 10.1016/j.neo.2014.08.004. Neoplasia. 2014. PMID: 25246273 Free PMC article.
-
Interactions of the Chemokine CCL5/RANTES with Medium-Sized Chondroitin Sulfate Ligands.Structure. 2015 Jun 2;23(6):1066-77. doi: 10.1016/j.str.2015.03.024. Epub 2015 May 14. Structure. 2015. PMID: 25982530 Free PMC article.
-
Contribution of the putative heparan sulfate-binding motif BBXB of RANTES to transendothelial migration.Glycobiology. 2002 Sep;12(9):535-43. doi: 10.1093/glycob/cwf069. Glycobiology. 2002. PMID: 12213786
-
The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients.Clin Cancer Res. 2006 Aug 1;12(15):4474-80. doi: 10.1158/1078-0432.CCR-06-0074. Clin Cancer Res. 2006. PMID: 16899591 Review.
-
CCL5's Role in Periodontal Disease: A Narrative Review.Int J Mol Sci. 2023 Dec 11;24(24):17332. doi: 10.3390/ijms242417332. Int J Mol Sci. 2023. PMID: 38139161 Free PMC article. Review.
Cited by
-
Overcoming intratumor heterogeneity of polygenic cancer drug resistance with improved biomarker integration.Neoplasia. 2012 Dec;14(12):1278-89. doi: 10.1593/neo.122096. Neoplasia. 2012. PMID: 23308059 Free PMC article.
-
Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers.Cancers (Basel). 2020 Jan 24;12(2):287. doi: 10.3390/cancers12020287. Cancers (Basel). 2020. PMID: 31991604 Free PMC article. Review.
-
The Recruitment of Neutrophils to the Tumor Microenvironment Is Regulated by Multiple Mediators.Front Immunol. 2021 Sep 10;12:734188. doi: 10.3389/fimmu.2021.734188. eCollection 2021. Front Immunol. 2021. PMID: 34567000 Free PMC article. Review.
-
The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization.Glycobiology. 2016 Mar;26(3):312-26. doi: 10.1093/glycob/cwv100. Epub 2015 Nov 17. Glycobiology. 2016. PMID: 26582609 Free PMC article.
-
Cancer subclonal genetic architecture as a key to personalized medicine.Neoplasia. 2013 Dec;15(12):1410-20. doi: 10.1593/neo.131972. Neoplasia. 2013. PMID: 24403863 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. - PubMed
-
- Soria G, Ben-Baruch A. The CCL5/CCR5 Axis in Cancer. New York, NY: Humana Press; 2009.
-
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
