Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis
- PMID: 22352884
- PMCID: PMC3323110
- DOI: 10.1021/bi201628j
Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis
Abstract
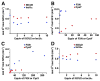
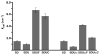
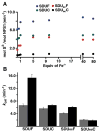
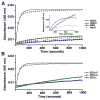
Human frataxin (FXN) has been intensively studied since the discovery that the FXN gene is associated with the neurodegenerative disease Friedreich's ataxia. Human FXN is a component of the NFS1-ISD11-ISCU2-FXN (SDUF) core Fe-S assembly complex and activates the cysteine desulfurase and Fe-S cluster biosynthesis reactions. In contrast, the Escherichia coli FXN homologue CyaY inhibits Fe-S cluster biosynthesis. To resolve this discrepancy, enzyme kinetic experiments were performed for the human and E. coli systems in which analogous cysteine desulfurase, Fe-S assembly scaffold, and frataxin components were interchanged. Surprisingly, our results reveal that activation or inhibition by the frataxin homologue is determined by which cysteine desulfurase is present and not by the identity of the frataxin homologue. These data are consistent with a model in which the frataxin-less Fe-S assembly complex exists as a mixture of functional and nonfunctional states, which are stabilized by binding of frataxin homologues. Intriguingly, this appears to be an unusual example in which modifications to an enzyme during evolution inverts or reverses the mode of control imparted by a regulatory molecule.
Figures


Similar articles
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
-
Mechanism of activation of the human cysteine desulfurase complex by frataxin.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19421-19430. doi: 10.1073/pnas.1909535116. Epub 2019 Sep 11. Proc Natl Acad Sci U S A. 2019. PMID: 31511419 Free PMC article.
-
Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry.Biochemistry. 2014 Aug 5;53(30):4904-13. doi: 10.1021/bi500532e. Epub 2014 Jul 18. Biochemistry. 2014. PMID: 24971490 Free PMC article.
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
-
Frataxin and mitochondrial FeS cluster biogenesis.J Biol Chem. 2010 Aug 27;285(35):26737-26743. doi: 10.1074/jbc.R110.118679. Epub 2010 Jun 3. J Biol Chem. 2010. PMID: 20522547 Free PMC article. Review.
Cited by
-
Zinc and the iron donor frataxin regulate oligomerization of the scaffold protein to form new Fe-S cluster assembly centers.Metallomics. 2017 Jun 21;9(6):773-801. doi: 10.1039/c7mt00089h. Metallomics. 2017. PMID: 28548666 Free PMC article.
-
Mechanistic Insights into IscU Conformation Regulation for Fe-S Cluster Biogenesis Revealed by Variable Temperature Electrospray Ionization Native Ion Mobility Mass Spectrometry.Biochemistry. 2022 Dec 6;61(23):2733-2741. doi: 10.1021/acs.biochem.2c00429. Epub 2022 Nov 9. Biochemistry. 2022. PMID: 36351081 Free PMC article.
-
Structural and functional characterization of a frataxin from a thermophilic organism.FEBS J. 2019 Feb;286(3):495-506. doi: 10.1111/febs.14750. Epub 2019 Jan 30. FEBS J. 2019. PMID: 30636112 Free PMC article.
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
-
Iron-sulfur clusters: from metals through mitochondria biogenesis to disease.J Biol Inorg Chem. 2018 Jun;23(4):509-520. doi: 10.1007/s00775-018-1548-6. Epub 2018 Mar 6. J Biol Inorg Chem. 2018. PMID: 29511832 Free PMC article. Review.
References
-
- Johnson DC, Dean DR, Smith AD, Johnson M. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Brzóska K, Meczyńska S, Kruszewski M. Iron-sulfur cluster proteins: electron transfer and beyond. Acta Biochim Pol. 2006;53:685–691. - PubMed
-
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. - PubMed
-
- Meyer J. Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J Biol Inorg Chem. 2008;13:157–170. - PubMed
-
- Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

