The PI3K/Akt pathway contributes to arenavirus budding
- PMID: 22345463
- PMCID: PMC3318602
- DOI: 10.1128/JVI.06604-11
The PI3K/Akt pathway contributes to arenavirus budding
Abstract
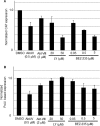
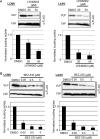
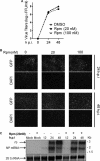
Several arenaviruses, chiefly Lassa virus (LASV), cause hemorrhagic fever (HF) disease in humans and pose a significant public health concern in regions where they are endemic. On the other hand, evidence indicates that the globally distributed prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) is a neglected human pathogen. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway participates in many cellular processes, including cell survival and differentiation, and also has been shown to play important roles in different steps of the life cycles of a variety of viruses. Here we report that the inhibition of the PI3K/Akt pathway inhibited budding and to a lesser extent RNA synthesis, but not cell entry, of LCMV. Accordingly, BEZ-235, a PI3K inhibitor currently in cancer clinical trials, inhibited LCMV multiplication in cultured cells. These findings, together with those previously reported for Junin virus (JUNV), indicate that targeting the PI3K/Akt pathway could represent a novel antiviral strategy to combat human-pathogenic arenaviruses.
Figures

Similar articles
-
Inhibition of the PI3K/Akt pathway by Ly294002 does not prevent establishment of persistent Junín virus infection in Vero cells.Arch Virol. 2015 Feb;160(2):469-75. doi: 10.1007/s00705-014-2298-6. Epub 2014 Dec 7. Arch Virol. 2015. PMID: 25488290 Free PMC article.
-
Participation of the phosphatidylinositol 3-kinase/Akt pathway in Junín virus replication in vitro.Virus Res. 2009 Oct;145(1):166-70. doi: 10.1016/j.virusres.2009.07.004. Epub 2009 Jul 10. Virus Res. 2009. PMID: 19595723 Free PMC article.
-
Identification and Mechanism of Action of a Novel Small-Molecule Inhibitor of Arenavirus Multiplication.J Virol. 2015 Nov;89(21):10924-33. doi: 10.1128/JVI.01587-15. Epub 2015 Aug 19. J Virol. 2015. Retraction in: J Virol. 2023 Mar 30;97(3):e0012323. doi: 10.1128/jvi.00123-23 PMID: 26292327 Free PMC article. Retracted.
-
Arenavirus reverse genetics: new approaches for the investigation of arenavirus biology and development of antiviral strategies.Virology. 2011 Mar 15;411(2):416-25. doi: 10.1016/j.virol.2011.01.013. Epub 2011 Feb 15. Virology. 2011. PMID: 21324503 Free PMC article. Review.
-
Arenavirus extinction through lethal mutagenesis.Virus Res. 2005 Feb;107(2):207-14. doi: 10.1016/j.virusres.2004.11.010. Virus Res. 2005. PMID: 15649566 Review.
Cited by
-
Molecular mechanism of arenavirus assembly and budding.Viruses. 2012 Oct 10;4(10):2049-79. doi: 10.3390/v4102049. Viruses. 2012. PMID: 23202453 Free PMC article. Review.
-
Cellular N-Myristoyl Transferases Are Required for Mammarenavirus Multiplication.Viruses. 2024 Aug 26;16(9):1362. doi: 10.3390/v16091362. Viruses. 2024. PMID: 39339839 Free PMC article.
-
The celecoxib derivative kinase inhibitor AR-12 (OSU-03012) inhibits Zika virus via down-regulation of the PI3K/Akt pathway and protects Zika virus-infected A129 mice: A host-targeting treatment strategy.Antiviral Res. 2018 Dec;160:38-47. doi: 10.1016/j.antiviral.2018.10.007. Epub 2018 Oct 13. Antiviral Res. 2018. PMID: 30326204 Free PMC article.
-
Hemorrhagic Fever-Causing Arenaviruses: Lethal Pathogens and Potent Immune Suppressors.Front Immunol. 2019 Mar 13;10:372. doi: 10.3389/fimmu.2019.00372. eCollection 2019. Front Immunol. 2019. PMID: 30918506 Free PMC article. Review.
-
A Highly Conserved Leucine in Mammarenavirus Matrix Z Protein Is Required for Z Interaction with the Virus L Polymerase and Z Stability in Cells Harboring an Active Viral Ribonucleoprotein.J Virol. 2018 May 14;92(11):e02256-17. doi: 10.1128/JVI.02256-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29593035 Free PMC article.
References
-
- Arita M, Wakita T, Shimizu H. 2009. Cellular kinase inhibitors that suppress enterovirus replication have a conserved target in viral protein 3A similar to that of enviroxime. J. Gen. Virol. 90:1869–1879 - PubMed
-
- Barton LL, Mets MB, Beauchamp CL. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187:1715–1716 - PubMed
-
- Battegay M. 1993. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 well plates. ALTEX 10:6–14 - PubMed
-
- Brognard J, Sierecki E, Gao T, Newton AC. 2007. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell 25:917–931 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

