Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p
- PMID: 22344582
- PMCID: PMC3286328
- DOI: 10.1093/brain/awr354
Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p
Abstract
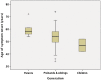
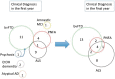
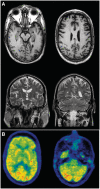
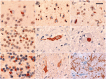
Frontotemporal dementia and amyotrophic lateral sclerosis are closely related clinical syndromes with overlapping molecular pathogenesis. Several families have been reported with members affected by frontotemporal dementia, amyotrophic lateral sclerosis or both, which show genetic linkage to a region on chromosome 9p21. Recently, two studies identified the FTD/ALS gene defect on chromosome 9p as an expanded GGGGCC hexanucleotide repeat in a non-coding region of the chromosome 9 open reading frame 72 gene (C9ORF72). In the present study, we provide detailed analysis of the clinical features and neuropathology for 16 unrelated families with frontotemporal dementia caused by the C9ORF72 mutation. All had an autosomal dominant pattern of inheritance. Eight families had a combination of frontotemporal dementia and amyotrophic lateral sclerosis while the other eight had a pure frontotemporal dementia phenotype. Clinical information was available for 30 affected members of the 16 families. There was wide variation in age of onset (mean = 54.3, range = 34-74 years) and disease duration (mean = 5.3, range = 1-16 years). Early diagnoses included behavioural variant frontotemporal dementia (n = 15), progressive non-fluent aphasia (n = 5), amyotrophic lateral sclerosis (n = 9) and progressive non-fluent aphasia-amyotrophic lateral sclerosis (n = 1). Heterogeneity in clinical presentation was also common within families. However, there was a tendency for the phenotypes to converge with disease progression; seven subjects had final clinical diagnoses of both frontotemporal dementia and amyotrophic lateral sclerosis and all of those with an initial progressive non-fluent aphasia diagnosis subsequently developed significant behavioural abnormalities. Twenty-one affected family members came to autopsy and all were found to have transactive response DNA binding protein with M(r) 43 kD (TDP-43) pathology in a wide neuroanatomical distribution. All had involvement of the extramotor neocortex and hippocampus (frontotemporal lobar degeneration-TDP) and all but one case (clinically pure frontotemporal dementia) had involvement of lower motor neurons, characteristic of amyotrophic lateral sclerosis. In addition, a consistent and relatively specific pathological finding was the presence of neuronal inclusions in the cerebellar cortex that were ubiquitin/p62-positive but TDP-43-negative. Our findings indicate that the C9ORF72 mutation is a major cause of familial frontotemporal dementia with TDP-43 pathology, that likely accounts for the majority of families with combined frontotemporal dementia/amyotrophic lateral sclerosis presentation, and further support the concept that frontotemporal dementia and amyotrophic lateral sclerosis represent a clinicopathological spectrum of disease with overlapping molecular pathogenesis.
Figures
Similar articles
-
Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72.Brain. 2012 Mar;135(Pt 3):765-83. doi: 10.1093/brain/aws004. Brain. 2012. PMID: 22366793 Free PMC article.
-
The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions.Brain. 2012 Mar;135(Pt 3):723-35. doi: 10.1093/brain/awr353. Epub 2012 Feb 1. Brain. 2012. PMID: 22300876
-
Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p.Acta Neuropathol. 2012 Mar;123(3):409-17. doi: 10.1007/s00401-011-0937-5. Epub 2012 Jan 7. Acta Neuropathol. 2012. PMID: 22228244 Free PMC article.
-
Prevalence of brain and spinal cord inclusions, including dipeptide repeat proteins, in patients with the C9ORF72 hexanucleotide repeat expansion: a systematic neuropathological review.Neuropathol Appl Neurobiol. 2016 Oct;42(6):547-60. doi: 10.1111/nan.12284. Neuropathol Appl Neurobiol. 2016. PMID: 26373655 Review.
-
[Overlapping features of frontotemporal dementia and amyotrophic lateral sclerosis].Rev Med Chil. 2014 Jul;142(7):867-79. doi: 10.4067/S0034-98872014000700007. Rev Med Chil. 2014. PMID: 25378006 Review. Spanish.
Cited by
-
A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats.Hum Mutat. 2013 Feb;34(2):363-73. doi: 10.1002/humu.22244. Epub 2013 Jan 4. Hum Mutat. 2013. PMID: 23111906 Free PMC article.
-
Genetics and Pathophysiology of Neurodegeneration with Brain Iron Accumulation (NBIA).Curr Neuropharmacol. 2013 Jan;11(1):59-79. doi: 10.2174/157015913804999469. Curr Neuropharmacol. 2013. PMID: 23814539 Free PMC article.
-
Modeling C9orf72-Related Frontotemporal Dementia and Amyotrophic Lateral Sclerosis in Drosophila.Front Cell Neurosci. 2021 Oct 21;15:770937. doi: 10.3389/fncel.2021.770937. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34744635 Free PMC article. Review.
-
C9orf72 mutation is rare in Alzheimer's disease, Parkinson's disease, and essential tremor in China.Front Cell Neurosci. 2013 Sep 24;7:164. doi: 10.3389/fncel.2013.00164. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24068985 Free PMC article.
-
Redox-mediated regulation of an evolutionarily conserved cross-β structure formed by the TDP43 low complexity domain.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):28727-28734. doi: 10.1073/pnas.2012216117. Epub 2020 Nov 3. Proc Natl Acad Sci U S A. 2020. PMID: 33144500 Free PMC article.
References
-
- Arai T, Mackenzie IRA, Hasegawa M, Nonoka Y, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–36. - PubMed
-
- Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. - PubMed
-
- Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

