Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia
- PMID: 22344033
- PMCID: PMC3290742
- DOI: 10.1038/ncb2432
Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia
Abstract
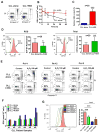
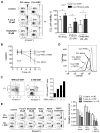
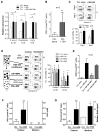
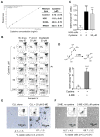
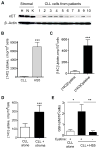
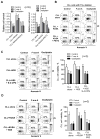
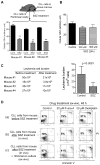
Tissue stromal cells interact with leukaemia cells and profoundly affect their viability and drug sensitivity. Here we show a biochemical mechanism by which bone marrow stromal cells modulate the redox status of chronic lymphocytic leukaemia (CLL) cells and promote cellular survival and drug resistance. Primary CLL cells from patients exhibit a limited ability to transport cystine for glutathione (GSH) synthesis owing to a low expression level of Xc-transporter. In contrast, bone marrow stromal cells effectively import cystine and convert it to cysteine, which is then released into the microenvironment for uptake by CLL cells to promote GSH synthesis. The elevated level of GSH enhances leukaemia cell survival and protects them from drug-induced cytotoxicity. Furthermore, disabling this protective mechanism significantly sensitizes CLL cells to drug treatment in the stromal environment. This stromal-leukaemia interaction is critical for CLL cell survival and represents a key biochemical pathway for effectively targeting leukaemia cells to overcome drug resistance in vivo.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Comment in
-
Good neighbours in the tumour stroma reduce oxidative stress.Nat Cell Biol. 2012 Feb 19;14(3):235-6. doi: 10.1038/ncb2449. Nat Cell Biol. 2012. PMID: 22344034
-
Leukaemia: Adding e for survival.Nat Rev Cancer. 2012 Mar 15;12(4):230. doi: 10.1038/nrc3249. Nat Rev Cancer. 2012. PMID: 22419254 No abstract available.
Similar articles
-
Cross-talk between chronic lymphocytic leukemia (CLL) tumor B cells and mesenchymal stromal cells (MSCs): implications for neoplastic cell survival.Oncotarget. 2015 Dec 8;6(39):42130-49. doi: 10.18632/oncotarget.6239. Oncotarget. 2015. PMID: 26517523 Free PMC article.
-
Bone marrow stromal cell-derived vascular endothelial growth factor (VEGF) rather than chronic lymphocytic leukemia (CLL) cell-derived VEGF is essential for the apoptotic resistance of cultured CLL cells.Mol Med. 2011;17(7-8):619-27. doi: 10.2119/molmed.2010.00210. Epub 2011 Apr 14. Mol Med. 2011. PMID: 21519633 Free PMC article.
-
Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance.Blood. 2009 Nov 12;114(20):4441-50. doi: 10.1182/blood-2009-07-233718. Epub 2009 Sep 17. Blood. 2009. PMID: 19762485 Free PMC article.
-
Modeling the chronic lymphocytic leukemia microenvironment in vitro.Leuk Lymphoma. 2017 Feb;58(2):266-279. doi: 10.1080/10428194.2016.1204654. Epub 2016 Oct 18. Leuk Lymphoma. 2017. PMID: 27756161 Review.
-
Interactions between bone marrow stromal microenvironment and B-chronic lymphocytic leukemia cells: any role for Notch, Wnt and Hh signaling pathways?Cell Signal. 2012 Jul;24(7):1433-43. doi: 10.1016/j.cellsig.2012.03.008. Epub 2012 Mar 13. Cell Signal. 2012. PMID: 22446006 Review.
Cited by
-
A Cystine-Cysteine Intercellular Shuttle Prevents Ferroptosis in xCTKO Pancreatic Ductal Adenocarcinoma Cells.Cancers (Basel). 2021 Mar 21;13(6):1434. doi: 10.3390/cancers13061434. Cancers (Basel). 2021. PMID: 33801101 Free PMC article.
-
Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance.Cancer Cell. 2014 Mar 17;25(3):350-65. doi: 10.1016/j.ccr.2014.02.005. Cancer Cell. 2014. PMID: 24651014 Free PMC article.
-
How will B-cell-receptor-targeted therapies change future CLL therapy?Blood. 2014 Mar 6;123(10):1455-60. doi: 10.1182/blood-2013-09-453092. Epub 2014 Jan 6. Blood. 2014. PMID: 24394667 Free PMC article. Review.
-
Targeting hexokinase 2 increases the sensitivity of oxaliplatin by Twist1 in colorectal cancer.J Cell Mol Med. 2021 Sep;25(18):8836-8849. doi: 10.1111/jcmm.16842. Epub 2021 Aug 10. J Cell Mol Med. 2021. PMID: 34378321 Free PMC article.
-
Proteomic identification of prognostic tumour biomarkers, using chemotherapy-induced cancer-associated fibroblasts.Aging (Albany NY). 2015 Oct;7(10):816-38. doi: 10.18632/aging.100808. Aging (Albany NY). 2015. PMID: 26539730 Free PMC article.
References
-
- Zwiebel JA, Cheson BD. Chronic lymphocytic leukemia: staging and prognostic factors. Seminars in oncology. 1998;25:42–59. - PubMed
-
- Keating MJ, et al. Biology and treatment of chronic lymphocytic leukemia. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. 2003:153–175. - PubMed
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–815. - PubMed
-
- Tam CS, Keating MJ. Chemoimmunotherapy of chronic lymphocytic leukemia. Best practice & research. 2007;20:479–498. - PubMed
-
- Rai KR, Chiorazzi N. Determining the clinical course and outcome in chronic lymphocytic leukemia. The New England journal of medicine. 2003;348:1797–1799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

