Ligation of cytotoxic T lymphocyte antigen-4 to T cell receptor inhibits T cell activation and directs differentiation into Foxp3+ regulatory T cells
- PMID: 22337882
- PMCID: PMC3322849
- DOI: 10.1074/jbc.M111.283705
Ligation of cytotoxic T lymphocyte antigen-4 to T cell receptor inhibits T cell activation and directs differentiation into Foxp3+ regulatory T cells
Abstract
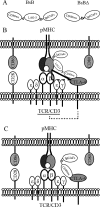
Cross-linking of ligand-engaged cytotoxic T lymphocyte antigen-4 (CTLA-4) to the T cell receptor (TCR) during the early phase of T cell activation attenuates TCR signaling, leading to T cell inhibition. To promote this event, a bispecific fusion protein comprising a mutant mouse CD80 (CD80w88a) and lymphocyte activation antigen-3 was engineered to concurrently engage CTLA-4 and cross-link it to the TCR. Cross-linking is expected to be attained via ligation of CTLA-4 first to MHCII and then indirectly to the TCR, generating a CTLA-4-MHCII-TCR trimolecular complex that forms between T cells and antigen-presenting cells during T cell activation. Treating T cells with this bispecific fusion protein inhibited T cell activation. In addition, it induced the production of IL-10 and TGF-β and attenuated AKT and mTOR signaling. Intriguingly, treatment with the bispecific fusion protein also directed early T cell differentiation into Foxp3-positive regulatory T cells (Tregs). This process was dependent on the endogenous production of TGF-β. Thus, bispecific fusion proteins that engage CTLA-4 and co-ligate it to the TCR during the early phase of T cell activation can negatively regulate the T cell response. Bispecific biologics with such dual functions may therefore represent a novel class of therapeutics for immune modulation. These findings presented here also reveal a potential new role for CTLA-4 in Treg differentiation.
Figures
Similar articles
-
A bispecific protein capable of engaging CTLA-4 and MHCII protects non-obese diabetic mice from autoimmune diabetes.PLoS One. 2013 May 21;8(5):e63530. doi: 10.1371/journal.pone.0063530. Print 2013. PLoS One. 2013. PMID: 23704916 Free PMC article.
-
Tumor Progression Locus 2 (Tpl2) Activates the Mammalian Target of Rapamycin (mTOR) Pathway, Inhibits Forkhead Box P3 (FoxP3) Expression, and Limits Regulatory T Cell (Treg) Immunosuppressive Functions.J Biol Chem. 2016 Aug 5;291(32):16802-15. doi: 10.1074/jbc.M116.718783. Epub 2016 Jun 3. J Biol Chem. 2016. PMID: 27261457 Free PMC article.
-
Integrated T-cell receptor and costimulatory signals determine TGF-β-dependent differentiation and maintenance of Foxp3+ regulatory T cells.Eur J Immunol. 2011 May;41(5):1242-8. doi: 10.1002/eji.201041073. Epub 2011 Apr 11. Eur J Immunol. 2011. PMID: 21469110
-
Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes.Immunology. 2020 May;160(1):24-37. doi: 10.1111/imm.13178. Epub 2020 Mar 9. Immunology. 2020. PMID: 32022254 Free PMC article. Review.
-
Transcriptional control of regulatory T cell development and function.Trends Immunol. 2013 Nov;34(11):531-9. doi: 10.1016/j.it.2013.08.003. Epub 2013 Sep 6. Trends Immunol. 2013. PMID: 24016547 Free PMC article. Review.
Cited by
-
Role of cytokines in thymus- versus peripherally derived-regulatory T cell differentiation and function.Front Immunol. 2013 Jun 19;4:155. doi: 10.3389/fimmu.2013.00155. eCollection 2013. Front Immunol. 2013. PMID: 23801992 Free PMC article.
-
Victory and defeat in the induction of a therapeutic response through vaccine therapy for human and canine brain tumors: a review of the state of the art.Crit Rev Immunol. 2014;34(5):399-432. doi: 10.1615/critrevimmunol.2014011577. Crit Rev Immunol. 2014. PMID: 25404047 Free PMC article. Review.
-
Epistasis and immunity: the role of genetic interactions in autoimmune diseases.Immunology. 2012 Oct;137(2):131-8. doi: 10.1111/j.1365-2567.2012.03623.x. Immunology. 2012. PMID: 22804709 Free PMC article. Review.
-
The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome.Front Immunol. 2015 Feb 18;6:61. doi: 10.3389/fimmu.2015.00061. eCollection 2015. Front Immunol. 2015. PMID: 25741338 Free PMC article. Review.
-
Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches.Breast Cancer (Dove Med Press). 2019 Jan 17;11:53-69. doi: 10.2147/BCTT.S175360. eCollection 2019. Breast Cancer (Dove Med Press). 2019. PMID: 30697064 Free PMC article. Review.
References
-
- Eagar T. N., Karandikar N. J., Bluestone J. A., Miller S. D. (2002) The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur. J. Immunol. 32, 972–981 - PubMed
-
- Tivol E. A., Borriello F., Schweitzer A. N., Lynch W. P., Bluestone J. A., Sharpe A. H. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 - PubMed
-
- Walunas T. L., Bluestone J. A. (1998) CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J. Immunol. 160, 3855–3860 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

