Immunotherapy earns its spot in the ranks of cancer therapy
- PMID: 22330682
- PMCID: PMC3280881
- DOI: 10.1084/jem.20112275
Immunotherapy earns its spot in the ranks of cancer therapy
Abstract
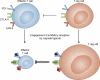
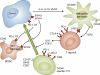
Since it became clear that all cancer cells express tumor-specific and tumor-selective antigens generated by genetic alterations and epigenetic dysregulation, the immunology community has embraced the possibility of designing therapies to induce targeted antitumor immune responses. The potential therapeutic specificity and efficacy of such treatments are obvious to anyone who studies the exquisite specificity and cytocidal potency of immune responses. However, the value assigned to a therapeutic modality by the oncology community at large does not depend on scientific principle; all that matters is how patients respond. The bar for the ultimate acceptance of a therapy requires more than anecdotal clinical responses; rather, the major modalities of cancer therapeutics, including surgery, chemotherapy, radiation therapy, and, more recently, drugs targeting oncogenes, have earned their place only after producing dramatic frequent clinical responses or demonstrating statistically significant survival benefits in large randomized phase 3 clinical trials, leading to FDA approval. Although tumor-targeted antibodies have certainly cleared this bar, immunotherapies aimed at harnessing antitumor cellular responses have not-until now.
Figures
Similar articles
-
The role of antigen-specific and non-specific immunotherapy in the treatment of cancer.J Immunotoxicol. 2012 Jul-Sep;9(3):248-58. doi: 10.3109/1547691X.2012.685527. Epub 2012 Jun 26. J Immunotoxicol. 2012. PMID: 22734880 Review.
-
Therapeutic cancer vaccines: From initial findings to prospects.Immunol Lett. 2018 Apr;196:11-21. doi: 10.1016/j.imlet.2018.01.011. Epub 2018 Feb 4. Immunol Lett. 2018. PMID: 29407608 Review.
-
Cancer immunotherapy: sipuleucel-T and beyond.Pharmacotherapy. 2011 Aug;31(8):813-28. doi: 10.1592/phco.31.8.813. Pharmacotherapy. 2011. PMID: 21923608 Free PMC article. Review.
-
Promising approaches in cancer immunotherapy.Immunobiology. 2020 Mar;225(2):151875. doi: 10.1016/j.imbio.2019.11.010. Epub 2019 Nov 29. Immunobiology. 2020. PMID: 31812343 Review.
-
Novel antibodies targeting immune regulatory checkpoints for cancer therapy.Br J Clin Pharmacol. 2013 Aug;76(2):233-47. doi: 10.1111/bcp.12164. Br J Clin Pharmacol. 2013. PMID: 23701301 Free PMC article. Review.
Cited by
-
SHP-1 phosphatase activity counteracts increased T cell receptor affinity.J Clin Invest. 2013 Mar;123(3):1044-56. doi: 10.1172/JCI65325. Epub 2013 Feb 8. J Clin Invest. 2013. PMID: 23391724 Free PMC article.
-
Recent updates on cancer immunotherapy.Precis Clin Med. 2018 Sep;1(2):65-74. doi: 10.1093/pcmedi/pby011. Epub 2018 Sep 6. Precis Clin Med. 2018. PMID: 30687562 Free PMC article. Review.
-
Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers.Onco Targets Ther. 2019 Apr 3;12:2513-2518. doi: 10.2147/OTT.S198421. eCollection 2019. Onco Targets Ther. 2019. PMID: 31040694 Free PMC article.
-
Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy.J Transl Med. 2016 Jun 1;14(1):155. doi: 10.1186/s12967-016-0910-0. J Transl Med. 2016. PMID: 27246873 Free PMC article.
-
Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer.Hum Pathol. 2013 Nov;44(11):2549-57. doi: 10.1016/j.humpath.2013.06.016. Epub 2013 Sep 26. Hum Pathol. 2013. PMID: 24074532 Free PMC article.
References
-
- Beck K.E., Blansfield J.A., Tran K.Q., Feldman A.L., Hughes M.S., Royal R.E., Kammula U.S., Topalian S.L., Sherry R.M., Kleiner D., et al. 2006. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 24:2283–2289 10.1200/JCO.2005.04.5716 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

