Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells
- PMID: 22326220
- PMCID: PMC3278719
- DOI: 10.1016/j.cmet.2012.01.009
Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells
Abstract
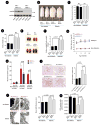
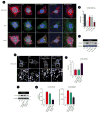
Several lines of evidence suggest that mitochondrial dysfunction plays a critical role in the pathogenesis of microvascular complications of diabetes, including diabetic nephropathy. However, the signaling pathways by which hyperglycemia leads to mitochondrial dysfunction are not fully understood. Here we examined the role of Rho-associated coiled coil-containing protein kinase 1 (ROCK1) on mitochondrial dynamics by generating two diabetic mouse models with targeted deletions of ROCK1 and an inducible podocyte-specific knockin mouse expressing a constitutively active (cA) mutant of ROCK1. Our findings suggest that ROCK1 mediates hyperglycemia-induced mitochondrial fission by promoting dynamin-related protein-1 (Drp1) recruitment to the mitochondria. Deletion of ROCK1 in diabetic mice prevented mitochondrial fission, whereas podocyte-specific cA-ROCK1 mice exhibited increased mitochondrial fission. Importantly, we found that ROCK1 triggers mitochondrial fission by phosphorylating Drp1 at serine 600 residue. These findings provide insights into the unexpected role of ROCK1 in a signaling cascade that regulates mitochondrial dynamics.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Increased thromboxane/prostaglandin receptors contribute to high glucose-induced podocyte injury and mitochondrial fission through ROCK1-Drp1 signaling.Int J Biochem Cell Biol. 2022 Oct;151:106281. doi: 10.1016/j.biocel.2022.106281. Epub 2022 Aug 20. Int J Biochem Cell Biol. 2022. PMID: 35995387
-
FOXO1 inhibition potentiates endothelial angiogenic functions in diabetes via suppression of ROCK1/Drp1-mediated mitochondrial fission.Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2481-2494. doi: 10.1016/j.bbadis.2018.04.005. Epub 2018 Apr 11. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29654945
-
Sirt6 deficiency contributes to mitochondrial fission and oxidative damage in podocytes via ROCK1-Drp1 signalling pathway.Cell Prolif. 2022 Oct;55(10):e13296. doi: 10.1111/cpr.13296. Epub 2022 Jul 17. Cell Prolif. 2022. PMID: 35842903 Free PMC article.
-
Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases.Brain Res Rev. 2011 Jun 24;67(1-2):103-18. doi: 10.1016/j.brainresrev.2010.11.004. Epub 2010 Dec 8. Brain Res Rev. 2011. PMID: 21145355 Free PMC article. Review.
-
Mitochondrial function in vascular endothelial cell in diabetes.J Smooth Muscle Res. 2012;48(1):1-26. doi: 10.1540/jsmr.48.1. J Smooth Muscle Res. 2012. PMID: 22504486 Free PMC article. Review.
Cited by
-
Targeting Methylglyoxal in Diabetic Kidney Disease Using the Mitochondria-Targeted Compound MitoGamide.Nutrients. 2021 Apr 25;13(5):1457. doi: 10.3390/nu13051457. Nutrients. 2021. PMID: 33922959 Free PMC article.
-
Effects of phosphorylation on Drp1 activation by its receptors, actin, and cardiolipin.Mol Biol Cell. 2024 Feb 1;35(2):ar16. doi: 10.1091/mbc.E23-11-0427. Epub 2023 Nov 29. Mol Biol Cell. 2024. PMID: 38019609 Free PMC article.
-
Mitochondrial network remodeling of the diabetic heart: implications to ischemia related cardiac dysfunction.Cardiovasc Diabetol. 2024 Jul 18;23(1):261. doi: 10.1186/s12933-024-02357-1. Cardiovasc Diabetol. 2024. PMID: 39026280 Free PMC article. Review.
-
Novel insights into the involvement of mitochondrial fission/fusion in heart failure: From molecular mechanisms to targeted therapies.Cell Stress Chaperones. 2023 Mar;28(2):133-144. doi: 10.1007/s12192-023-01321-4. Epub 2023 Jan 18. Cell Stress Chaperones. 2023. PMID: 36652120 Free PMC article. Review.
-
MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury.J Clin Invest. 2018 Dec 3;128(12):5448-5464. doi: 10.1172/JCI121859. Epub 2018 Nov 12. J Clin Invest. 2018. PMID: 30325740 Free PMC article.
References
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
-
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007a;282:21583–21587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

