Changes in antigen-specific T-cell number and function during oral desensitization in cow's milk allergy enabled with omalizumab
- PMID: 22318492
- PMCID: PMC3328586
- DOI: 10.1038/mi.2012.5
Changes in antigen-specific T-cell number and function during oral desensitization in cow's milk allergy enabled with omalizumab
Abstract
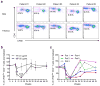
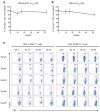
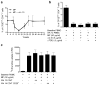
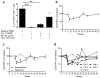
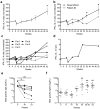
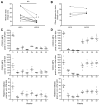
Food allergy is a major public health problem, for which there is no effective treatment. We examined the immunological changes that occurred in a group of children with significant cow's milk allergy undergoing a novel and rapid high-dose oral desensitization protocol enabled by treatment with omalizumab (anti-immunoglobulin (Ig)E monoclonal antibodies). Within a week of treatment, the CD4(+) T-cell response to milk was nearly eliminated, suggesting anergy in, or deletion of, milk-specific CD4(+) T cells. Over the following 3 months while the subjects remained on high doses of daily oral milk, the CD4(+) T-cell response returned, characterized by a shift from interleukin-4 to interferon-γ production. Desensitization was also associated with reduction in milk-specific IgE and a 15-fold increase in milk-specific IgG4. These studies suggest that high-dose oral allergen desensitization may be associated with deletion of allergen-specific T cells, without the apparent development of allergen-specific Foxp3(+) regulatory T cells.
Trial registration: ClinicalTrials.gov NCT00968110.
Conflict of interest statement
Figures
Similar articles
-
Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy.J Allergy Clin Immunol. 2011 Jun;127(6):1622-4. doi: 10.1016/j.jaci.2011.04.009. Epub 2011 May 4. J Allergy Clin Immunol. 2011. PMID: 21546071 Free PMC article. Clinical Trial. No abstract available.
-
Successful Milk Oral Immunotherapy Promotes Generation of Casein-Specific CD137+ FOXP3+ Regulatory T Cells Detectable in Peripheral Blood.Front Immunol. 2021 Nov 23;12:705615. doi: 10.3389/fimmu.2021.705615. eCollection 2021. Front Immunol. 2021. PMID: 34887847 Free PMC article.
-
Efficacy and safety of oral desensitization in children with cow's milk allergy according to their serum specific IgE level.Ann Allergy Asthma Immunol. 2013 Apr;110(4):290-4. doi: 10.1016/j.anai.2013.01.013. Epub 2013 Feb 14. Ann Allergy Asthma Immunol. 2013. PMID: 23535095 Clinical Trial.
-
Immunotherapy for cow's milk allergy.Hum Vaccin Immunother. 2017 Oct 3;13(10):2443-2451. doi: 10.1080/21645515.2017.1353845. Hum Vaccin Immunother. 2017. PMID: 28825866 Free PMC article. Review.
-
Oral desensitization with cow's milk in IgE-mediated cow's milk allergy. Contra!Monogr Allergy. 1996;32:233-5. Monogr Allergy. 1996. PMID: 8813207 Review. No abstract available.
Cited by
-
Mechanisms of Oral Tolerance.Clin Rev Allergy Immunol. 2018 Oct;55(2):107-117. doi: 10.1007/s12016-018-8680-5. Clin Rev Allergy Immunol. 2018. PMID: 29488131 Free PMC article. Review.
-
Enhancing the Safety and Efficacy of Food Allergy Immunotherapy: a Review of Adjunctive Therapies.Clin Rev Allergy Immunol. 2018 Oct;55(2):172-189. doi: 10.1007/s12016-018-8694-z. Clin Rev Allergy Immunol. 2018. PMID: 29968170 Review.
-
Oral Food Desensitization in Children With IgE-Mediated Cow's Milk Allergy: Immunological Changes Underlying Desensitization.Allergy Asthma Immunol Res. 2017 Jan;9(1):35-42. doi: 10.4168/aair.2017.9.1.35. Allergy Asthma Immunol Res. 2017. PMID: 27826960 Free PMC article.
-
Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells: potential effects on the tumor microenvironment.Cancer Biol Ther. 2014 Apr;15(4):409-18. doi: 10.4161/cbt.27627. Epub 2014 Jan 14. Cancer Biol Ther. 2014. PMID: 24423651 Free PMC article.
-
Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma.Allergy. 2020 Dec;75(12):3039-3068. doi: 10.1111/all.14582. Epub 2020 Sep 30. Allergy. 2020. PMID: 32893900 Free PMC article. Review.
References
-
- Noon L. Prophylactic inoculation for hay fever. Lancet. 1911;1:1572.
-
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. - PubMed
-
- Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011 in press. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

