The human respiratory syncytial virus matrix protein is required for maturation of viral filaments
- PMID: 22318136
- PMCID: PMC3318654
- DOI: 10.1128/JVI.06744-11
The human respiratory syncytial virus matrix protein is required for maturation of viral filaments
Abstract
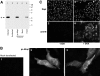
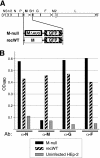
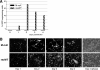
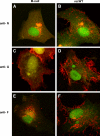
An experimental system was developed to generate infectious human respiratory syncytial virus (HRSV) lacking matrix (M) protein expression (M-null virus) from cDNA. The role of the M protein in virus assembly was then examined by infecting HEp-2 and Vero cells with the M-null virus and assessing the impact on infectious virus production and viral protein trafficking. In the absence of M, the production of infectious progeny was strongly impaired. Immunofluorescence (IF) microscopy analysis using antibodies against the nucleoprotein (N), attachment protein (G), and fusion protein (F) failed to detect the characteristic virus-induced cell surface filaments, which are believed to represent infectious virions. In addition, a large proportion of the N protein was detected in viral replication factories termed inclusion bodies (IBs). High-resolution analysis of the surface of M-null virus-infected cells by field emission scanning electron microscopy (SEM) revealed the presence of large areas with densely packed, uniformly short filaments. Although unusually short, these filaments were otherwise similar to those induced by an M-containing control virus, including the presence of the viral G and F proteins. The abundance of the short, stunted filaments in the absence of M indicates that M is not required for the initial stages of filament formation but plays an important role in the maturation or elongation of these structures. In addition, the absence of mature viral filaments and the simultaneous increase in the level of the N protein within IBs suggest that the M protein is involved in the transport of viral ribonucleoprotein (RNP) complexes from cytoplasmic IBs to sites of budding.
Figures
Similar articles
-
The respiratory syncytial virus fusion protein targets to the perimeter of inclusion bodies and facilitates filament formation by a cytoplasmic tail-dependent mechanism.J Virol. 2013 Oct;87(19):10730-41. doi: 10.1128/JVI.03086-12. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903836 Free PMC article.
-
The Respiratory Syncytial Virus Phosphoprotein, Matrix Protein, and Fusion Protein Carboxy-Terminal Domain Drive Efficient Filamentous Virus-Like Particle Formation.J Virol. 2016 Nov 14;90(23):10612-10628. doi: 10.1128/JVI.01193-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654298 Free PMC article.
-
trans-Complementation allows recovery of human respiratory syncytial viruses that are infectious but deficient in cell-to-cell transmission.J Virol. 2004 Sep;78(17):9064-72. doi: 10.1128/JVI.78.17.9064-9072.2004. J Virol. 2004. PMID: 15308702 Free PMC article.
-
Structural Insights into the Respiratory Syncytial Virus RNA Synthesis Complexes.Viruses. 2021 May 5;13(5):834. doi: 10.3390/v13050834. Viruses. 2021. PMID: 34063087 Free PMC article. Review.
-
Modulation of Host Immunity by Human Respiratory Syncytial Virus Virulence Factors: A Synergic Inhibition of Both Innate and Adaptive Immunity.Front Cell Infect Microbiol. 2017 Aug 16;7:367. doi: 10.3389/fcimb.2017.00367. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28861397 Free PMC article. Review.
Cited by
-
Function and Modulation of Type I Interferons during Respiratory Syncytial Virus Infection.Vaccines (Basel). 2020 Apr 10;8(2):177. doi: 10.3390/vaccines8020177. Vaccines (Basel). 2020. PMID: 32290326 Free PMC article. Review.
-
Paramyxovirus glycoprotein incorporation, assembly and budding: a three way dance for infectious particle production.Viruses. 2014 Aug 7;6(8):3019-54. doi: 10.3390/v6083019. Viruses. 2014. PMID: 25105277 Free PMC article. Review.
-
Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation.Cureus. 2023 Mar 18;15(3):e36342. doi: 10.7759/cureus.36342. eCollection 2023 Mar. Cureus. 2023. PMID: 37082497 Free PMC article. Review.
-
Respiratory syncytial virus assembles into structured filamentous virion particles independently of host cytoskeleton and related proteins.PLoS One. 2012;7(7):e40826. doi: 10.1371/journal.pone.0040826. Epub 2012 Jul 13. PLoS One. 2012. PMID: 22808269 Free PMC article.
-
The role of respiratory syncytial virus G protein in immune cell infection and pathogenesis.EBioMedicine. 2024 Sep;107:105318. doi: 10.1016/j.ebiom.2024.105318. Epub 2024 Aug 31. EBioMedicine. 2024. PMID: 39217853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

