Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases
- PMID: 22311979
- PMCID: PMC3322991
- DOI: 10.1074/jbc.M111.318022
Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases
Abstract
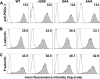
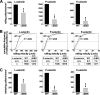
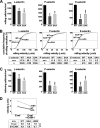
P-selectin glycoprotein ligand-1 (PSGL-1) mediates the capture (tethering) of free-flowing leukocytes and subsequent rolling on selectins. PSGL-1 interactions with endothelial selectins activate Src kinases and spleen tyrosine kinase (Syk), leading to α(L)β(2) integrin-dependent leukocyte slow rolling, which promotes leukocyte recruitment into tissues. In addition, but through a distinct pathway, PSGL-1 engagement activates ERK. Because ezrin, radixin and moesin proteins (ERMs) link PSGL-1 to actin cytoskeleton and because they serve as adaptor molecules between PSGL-1 and Syk, we examined the role of PSGL-1 ERM-binding sequence (EBS) on cell capture, rolling, and signaling through Syk and MAPK pathways. We carried out mutational analysis and observed that deletion of EBS severely reduced 32D leukocyte tethering and rolling on L-, P-, and E-selectin and slightly increased rolling velocity. Alanine substitution of Arg-337 and Lys-338 showed that these residues play a key role in supporting leukocyte tethering and rolling on selectins. Importantly, EBS deletion or Arg-337 and Lys-338 mutations abrogated PSGL-1-induced ERK activation, whereas they did not prevent Syk phosphorylation or E-selectin-induced leukocyte slow rolling. These studies demonstrate that PSGL-1 EBS plays a critical role in recruiting leukocytes on selectins and in activating the MAPK pathway, whereas it is dispensable to phosphorylate Syk and to lead to α(L)β(2)-dependent leukocyte slow rolling.
Figures

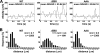

Similar articles
-
PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling.J Exp Med. 2008 Sep 29;205(10):2339-47. doi: 10.1084/jem.20072660. Epub 2008 Sep 15. J Exp Med. 2008. PMID: 18794338 Free PMC article.
-
ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1.Immunity. 2002 Oct;17(4):401-12. doi: 10.1016/s1074-7613(02)00420-x. Immunity. 2002. PMID: 12387735
-
Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin αLβ2.J Biol Chem. 2012 Jun 1;287(23):19585-98. doi: 10.1074/jbc.M112.361519. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511754 Free PMC article.
-
PSGL-1 function in immunity and steady state homeostasis.Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review.
-
Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways.Int Immunopharmacol. 2013 Dec;17(4):1185-97. doi: 10.1016/j.intimp.2013.11.010. Epub 2013 Nov 18. Int Immunopharmacol. 2013. PMID: 24263067 Review.
Cited by
-
PSGL-1 Immune Checkpoint Inhibition for CD4+ T Cell Cancer Immunotherapy.Front Immunol. 2021 Feb 23;12:636238. doi: 10.3389/fimmu.2021.636238. eCollection 2021. Front Immunol. 2021. PMID: 33708224 Free PMC article. Review.
-
Constitutive PSGL-1 Correlates with CD30 and TCR Pathways and Represents a Potential Target for Immunotherapy in Anaplastic Large T-Cell Lymphoma.Cancers (Basel). 2021 Jun 12;13(12):2958. doi: 10.3390/cancers13122958. Cancers (Basel). 2021. PMID: 34204843 Free PMC article.
-
Ezrin's role in gastric cancer progression: Implications for immune microenvironment modulation and therapeutic potential.Heliyon. 2024 Feb 28;10(5):e27155. doi: 10.1016/j.heliyon.2024.e27155. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38449647 Free PMC article.
-
Neutrophil arrest by LFA-1 activation.Front Immunol. 2012 Jun 12;3:157. doi: 10.3389/fimmu.2012.00157. eCollection 2012. Front Immunol. 2012. PMID: 22701459 Free PMC article.
-
Interactome analysis reveals ezrin can adopt multiple conformational states.J Biol Chem. 2013 Dec 6;288(49):35437-51. doi: 10.1074/jbc.M113.505669. Epub 2013 Oct 22. J Biol Chem. 2013. PMID: 24151071 Free PMC article.
References
-
- Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation. The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 - PubMed
-
- Sperandio M., Forlow S. B., Thatte J., Ellies L. G., Marth J. D., Ley K. (2001) Differential requirements for core2 glucosaminyltransferase for endothelial L-selectin ligand function in vivo. J. Immunol. 167, 2268–2274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

