Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor
- PMID: 22310931
- PMCID: PMC11029748
- DOI: 10.1007/s00262-012-1212-x
Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor
Abstract
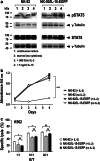
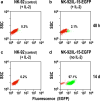
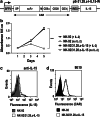
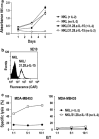
Natural killer (NK) cells hold promise for adoptive cancer immunotherapy but are dependent on cytokines such as interleukin (IL)-2 for growth and cytotoxicity. Here, we investigated the consequences of ectopic expression of IL-15 in human NK cells. IL-2 and IL-15 belong to the common γ chain family of cytokines and have overlapping activities. Transduction of clinically applicable NK-92 cells with lentiviral vectors encoding human IL-15 resulted in predominantly intracellular expression of the cytokine, and STAT5 activation, proliferation and cytotoxicity of the producer cells in the absence of IL-2. Growth of non-transduced bystander cells was not supported, allowing rapid enrichment of gene-modified cells solely by IL-2 withdrawal. This was also the case upon transduction of NK-92 and NKL cells with a bicistronic lentiviral vector encoding IL-15 and a chimeric antigen receptor (CAR) targeting the pancarcinoma antigen EpCAM. Effector cells co-expressing CAR and IL-15 continued to proliferate in the absence of exogenous cytokines and displayed high and selective cell-killing activity against EpCAM-expressing breast carcinoma cells that were resistant to the natural cytotoxicity of unmodified NK cells. This strategy facilitates rapid isolation and continuous expansion of retargeted NK cells and may extend their potential clinical utility.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15.Blood. 2014 Aug 14;124(7):1081-8. doi: 10.1182/blood-2014-02-556837. Epub 2014 Jul 8. Blood. 2014. PMID: 25006133
-
Efficient generation of gene-modified human natural killer cells via alpharetroviral vectors.J Mol Med (Berl). 2016 Jan;94(1):83-93. doi: 10.1007/s00109-015-1327-6. Epub 2015 Aug 25. J Mol Med (Berl). 2016. PMID: 26300042
-
Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells.Hum Gene Ther. 2017 Oct;28(10):897-913. doi: 10.1089/hum.2017.157. Epub 2017 Aug 15. Hum Gene Ther. 2017. PMID: 28810809
-
Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy.Acta Pharmacol Sin. 2018 Feb;39(2):167-176. doi: 10.1038/aps.2017.125. Epub 2017 Sep 7. Acta Pharmacol Sin. 2018. PMID: 28880014 Free PMC article. Review.
-
The regulation and biological activity of interleukin 12.Leuk Lymphoma. 1998 May;29(5-6):427-38. doi: 10.3109/10428199809050903. Leuk Lymphoma. 1998. PMID: 9643557 Review.
Cited by
-
Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival.Oncoimmunology. 2015 Dec 21;5(4):e1119354. doi: 10.1080/2162402X.2015.1119354. eCollection 2016 Apr. Oncoimmunology. 2015. PMID: 27141401 Free PMC article.
-
CAR-NK as a Rapidly Developed and Efficient Immunotherapeutic Strategy against Cancer.Cancers (Basel). 2022 Dec 24;15(1):117. doi: 10.3390/cancers15010117. Cancers (Basel). 2022. PMID: 36612114 Free PMC article. Review.
-
NK Cells Armed with Chimeric Antigen Receptors (CAR): Roadblocks to Successful Development.Cells. 2021 Dec 1;10(12):3390. doi: 10.3390/cells10123390. Cells. 2021. PMID: 34943898 Free PMC article. Review.
-
CAR-Based Strategies beyond T Lymphocytes: Integrative Opportunities for Cancer Adoptive Immunotherapy.Int J Mol Sci. 2019 Jun 11;20(11):2839. doi: 10.3390/ijms20112839. Int J Mol Sci. 2019. PMID: 31212634 Free PMC article. Review.
-
Engineered Adoptive T-Cell Therapies for Breast Cancer: Current Progress, Challenges, and Potential.Cancers (Basel). 2023 Dec 26;16(1):124. doi: 10.3390/cancers16010124. Cancers (Basel). 2023. PMID: 38201551 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

