A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis
- PMID: 22307873
- PMCID: PMC3376847
- DOI: 10.1002/emmm.201100200
A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis
Abstract
Endometriosis is found in 5-15% of women of reproductive age and is more frequent in relatives of women with the disease. Activation of KRAS results in de novo endometriosis in mice, however, activating KRAS mutations have not been identified in women. We screened 150 women with endometriosis for a polymorphism in a let-7 microRNA (miRNA) binding site in the 3'-UTR of KRAS and detected a KRAS variant allele in 31% of women with endometriosis as opposed to 5% of a large diverse control population. KRAS mRNA and protein expression were increased in cultured endometrial stromal cells of women with the KRAS variant. Increased KRAS protein was due to altered miRNA binding as demonstrated in reporter assays. Endometrial stromal cells from women with the KRAS variant showed increased proliferation and invasion. In a murine model, endometrial xenografts containing the KRAS variant demonstrated increased proliferation and decreased progesterone receptor levels. These findings suggest that an inherited polymorphism of a let-7 miRNA binding site in KRAS leads to abnormal endometrial growth and endometriosis. The LCS6 polymorphism is the first described genetic marker of endometriosis risk.
Copyright © 2012 EMBO Molecular Medicine.
Figures

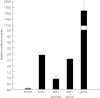
q-RT-PCR results show comparatively low levels of KRAS mRNA in normal endometrium, increased KRAS mRNA in hESC from women with endometriosis and the WT KRAS allele (p = 0.0007) and highest expression of KRAS mRNA in hESC carrying the variant KRAS allele (p = 0.00018 when compared to normal hESC, p = 0.0049 when compared to hESC from endometriosis patients with WT allele). (*, difference is significant when compared to normal hESC; **, difference is significant when compared to hESC from women with endometriosis homozygous for the WT KRAS allele).
Western blot results show a 2.8-fold increase in KRAS protein in hESC with the variant allele.

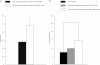
The effect of the KRAS variant allele on proliferation of hESC from women with endometriosis as determined by BrdU incorporation. There was a 71% increase (*p = 0.04) in the BrdU label in hESC with the variant allele (n = 5) versus WT KRAS LCS6 (n = 5). These results indicated an increase in cell proliferation rate of hESC containing the mutant KRAS LCS6.
The effect of the KRAS variant allele on hESC invasion capacity. An invasion assay in which hESC from women with and without endometriosis and with the WT non-variant or the alternative KRAS allele was used to determine the ability to invade extracellular matrix. There was a significant increase in the invasion of hESC containing the variant allele (n = 9) compared to hESC without the variant allele (n = 6) (*p = 0.013). The difference between normal cells (n = 6) and cells from endometriosis with WT KRAS was not significant.

Morphological appearance of the lesions under the kidney capsule of immune deficient mice 1 month after transplantation of hESC either with or without the variant KRAS. In all cases the transplanted endometrial cells formed endometriosis lesions with both glandular and stromal components.
Proliferation marker expression in endometriotic lesions in mice. Nuclear staining for PCNA was more prominent in epithelium and stroma of the lesions formed by KRAS variant positive cells (54 ± 5% and 56 ± 6% in epithelium and stroma, respectively), compared to those derived from normal cells (8 ± 4% and 34 ± 6% in epithelium and stroma, respectively; p = 0.02 and p = 0.043) indicating higher proliferation levels in these cells.
PR expression in endometriotic lesions with WT non-variant or variant KRAS allele in mice. Lesions created by hESC carrying KRAS variant allele were characterized by a smaller number of nuclei stained positively for PR in both glandular and stromal cells. The epithelium of the lesions with variant cells was found to have only 35 ± 5% of nuclei positively stained compared to 75 ± 3%, in the lesions with WT KRAS (p = 0.02). Only 13 ± 8% of nuclei of stromal cells in KRAS variant lesions were found to express PR compared to 78 ± 7% in the non-variant lesions (p = 0.028). Scale bar represents 25 µm.
Similar articles
-
Evaluation of KRAS Gene Expression and LCS6 Variant in Genomic and Cell-Free DNA of Iranian Women With Endometriosis.Reprod Sci. 2015 Jun;22(6):679-84. doi: 10.1177/1933719114556478. Epub 2014 Oct 30. Reprod Sci. 2015. PMID: 25361550 Free PMC article.
-
Association study of the let-7 miRNA-complementary site variant in the 3' untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial).Clin Cancer Res. 2014 Jun 15;20(12):3319-27. doi: 10.1158/1078-0432.CCR-14-0069. Epub 2014 Apr 11. Clin Cancer Res. 2014. PMID: 24727325 Free PMC article. Clinical Trial.
-
A let-7 microRNA-binding site polymorphism in the KRAS 3' UTR is associated with reduced survival in oral cancers.Carcinogenesis. 2009 Jun;30(6):1003-7. doi: 10.1093/carcin/bgp099. Epub 2009 Apr 20. Carcinogenesis. 2009. PMID: 19380522 Free PMC article.
-
Let-7 microRNA-binding-site polymorphism in the 3'UTR of KRAS and colorectal cancer outcome: a systematic review and meta-analysis.Cancer Med. 2014 Oct;3(5):1385-95. doi: 10.1002/cam4.279. Epub 2014 Jun 2. Cancer Med. 2014. PMID: 24890702 Free PMC article. Review.
-
MicroRNA-mediated regulation of KRAS in cancer.J Hematol Oncol. 2014 Nov 30;7:84. doi: 10.1186/s13045-014-0084-2. J Hematol Oncol. 2014. PMID: 25433809 Free PMC article. Review.
Cited by
-
No clinical utility of KRAS variant rs61764370 for ovarian or breast cancer.Gynecol Oncol. 2016 May;141(2):386-401. doi: 10.1016/j.ygyno.2015.04.034. Epub 2015 May 2. Gynecol Oncol. 2016. PMID: 25940428 Free PMC article. Review.
-
The Origin and Pathogenesis of Endometriosis.Annu Rev Pathol. 2020 Jan 24;15:71-95. doi: 10.1146/annurev-pathmechdis-012419-032654. Epub 2019 Sep 3. Annu Rev Pathol. 2020. PMID: 31479615 Free PMC article. Review.
-
Endometriosis.Endocr Rev. 2019 Aug 1;40(4):1048-1079. doi: 10.1210/er.2018-00242. Endocr Rev. 2019. PMID: 30994890 Free PMC article. Review.
-
Evaluation of KRAS Gene Expression and LCS6 Variant in Genomic and Cell-Free DNA of Iranian Women With Endometriosis.Reprod Sci. 2015 Jun;22(6):679-84. doi: 10.1177/1933719114556478. Epub 2014 Oct 30. Reprod Sci. 2015. PMID: 25361550 Free PMC article.
-
Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis.Fertil Steril. 2016 Sep 1;106(3):673-80. doi: 10.1016/j.fertnstert.2016.05.020. Epub 2016 Jun 16. Fertil Steril. 2016. PMID: 27320036 Free PMC article.
References
-
- Amemiya S, Sekizawa A, Otsuka J, Tachikawa T, Saito H, Okai T. Malignant transformation of endometriosis and genetic alterations of KRAS and microsatellite instability. Int J Gynecol Obstet. 2004;86:371–376. - PubMed
-
- Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. - PubMed
-
- Bulun SE. Mechanisms of disease endometriosis. N Engl J Med. 2009;360:268–279. - PubMed
-
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

