A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer
- PMID: 22305568
- PMCID: PMC3277444
- DOI: 10.1016/j.stem.2011.12.018
A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer
Abstract
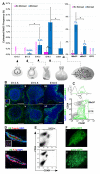
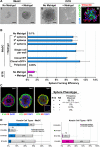
Gene expression signatures relating mammary stem cell populations to breast cancers have focused on adult tissue. Here, we identify, isolate, and characterize the fetal mammary stem cell (fMaSC) state since the invasive and proliferative processes of mammogenesis resemble phases of cancer progression. fMaSC frequency peaks late in embryogenesis, enabling more extensive stem cell purification than achieved with adult tissue. fMaSCs are self-renewing, multipotent, and coexpress multiple mammary lineage markers. Gene expression, transplantation, and in vitro analyses reveal putative autocrine and paracrine regulatory mechanisms, including ErbB and FGF signaling pathways impinging on fMaSC growth. Expression profiles from fMaSCs and associated stroma exhibit significant similarities to basal-like and Her2+ intrinsic breast cancer subtypes. Our results reveal links between development and cancer and provide resources to identify new candidates for diagnosis, prognosis, and therapy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Mammary development and breast cancer: the role of stem cells.Curr Mol Med. 2011 Jun;11(4):270-85. doi: 10.2174/156652411795678007. Curr Mol Med. 2011. PMID: 21506923 Free PMC article. Review.
-
Characterization of cell lines derived from breast cancers and normal mammary tissues for the study of the intrinsic molecular subtypes.Breast Cancer Res Treat. 2013 Nov;142(2):237-55. doi: 10.1007/s10549-013-2743-3. Epub 2013 Oct 27. Breast Cancer Res Treat. 2013. PMID: 24162158 Free PMC article.
-
Normal and cancerous mammary stem cells evade interferon-induced constraint through the miR-199a-LCOR axis.Nat Cell Biol. 2017 Jun;19(6):711-723. doi: 10.1038/ncb3533. Epub 2017 May 22. Nat Cell Biol. 2017. PMID: 28530657 Free PMC article.
-
Developmental signaling pathways regulating mammary stem cells and contributing to the etiology of triple-negative breast cancer.Breast Cancer Res Treat. 2016 Apr;156(2):211-26. doi: 10.1007/s10549-016-3746-7. Epub 2016 Mar 11. Breast Cancer Res Treat. 2016. PMID: 26968398 Free PMC article. Review.
-
Sox10 Regulates Stem/Progenitor and Mesenchymal Cell States in Mammary Epithelial Cells.Cell Rep. 2015 Sep 29;12(12):2035-48. doi: 10.1016/j.celrep.2015.08.040. Epub 2015 Sep 10. Cell Rep. 2015. PMID: 26365194 Free PMC article.
Cited by
-
Cbx8 Acts Non-canonically with Wdr5 to Promote Mammary Tumorigenesis.Cell Rep. 2016 Jul 12;16(2):472-486. doi: 10.1016/j.celrep.2016.06.002. Epub 2016 Jun 23. Cell Rep. 2016. PMID: 27346354 Free PMC article.
-
Low expression of galectin-3 is associated with poor survival in node-positive breast cancers and mesenchymal phenotype in breast cancer stem cells.Breast Cancer Res. 2016 Sep 29;18(1):97. doi: 10.1186/s13058-016-0757-6. Breast Cancer Res. 2016. PMID: 27687248 Free PMC article.
-
Gpr125 is a unifying hallmark of multiple mammary progenitors coupled to tumor latency.Nat Commun. 2022 Mar 17;13(1):1421. doi: 10.1038/s41467-022-28937-x. Nat Commun. 2022. PMID: 35302059 Free PMC article.
-
Maternal metabolic perturbations elicited by high-fat diet promote Wnt-1-induced mammary tumor risk in adult female offspring via long-term effects on mammary and systemic phenotypes.Carcinogenesis. 2014 Sep;35(9):2102-12. doi: 10.1093/carcin/bgu106. Epub 2014 May 15. Carcinogenesis. 2014. PMID: 24832086 Free PMC article.
-
Prenatal morphogenesis of mammary glands in mouse and rabbit.J Mammary Gland Biol Neoplasia. 2013 Jun;18(2):93-104. doi: 10.1007/s10911-013-9298-0. Epub 2013 Jun 5. J Mammary Gland Biol Neoplasia. 2013. PMID: 23736987 Free PMC article. Review.
References
-
- Anbazhagan R, Osin PP, Bartkova J, Nathan B, Lane EB, Gusterson BA. The development of epithelial phenotypes in the human fetal and infant breast. J Pathol. 1998;184:197–206. - PubMed
-
- Bonnefoix T, Bonnefoix P, Verdiel P, Sotto JJ. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J Immunol Methods. 1996;194:113–119. - PubMed
-
- Brewer BG, Mitchell RA, Harandi A, Eaton JW. Embryonic vaccines against cancer: an early history. Exp Mol Pathol. 2009;86:192–197. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

