DNA methylation signatures in development and aging of the human prefrontal cortex
- PMID: 22305529
- PMCID: PMC3276664
- DOI: 10.1016/j.ajhg.2011.12.020
DNA methylation signatures in development and aging of the human prefrontal cortex
Erratum in
- Am J Hum Genet. 2012 Oct 5;91(4):765
Abstract
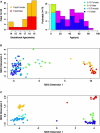
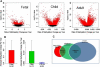
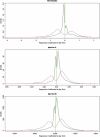
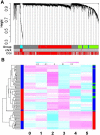
The human prefrontal cortex (PFC), a mastermind of the brain, is one of the last brain regions to mature. To investigate the role of epigenetics in the development of PFC, we examined DNA methylation in ∼14,500 genes at ∼27,000 CpG loci focused on 5' promoter regions in 108 subjects range in age from fetal to elderly. DNA methylation in the PFC shows unique temporal patterns across life. The fastest changes occur during the prenatal period, slow down markedly after birth and continue to slow further with aging. At the genome level, the transition from fetal to postnatal life is typified by a reversal of direction, from demethylation prenatally to increased methylation postnatally. DNA methylation is strongly associated with genotypic variants and correlates with expression of a subset of genes, including genes involved in brain development and in de novo DNA methylation. Our results indicate that promoter DNA methylation in the human PFC is a highly dynamic process modified by genetic variance and regulating gene transcription. Additional discovery is made possible with a stand-alone application, BrainCloudMethyl.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Illuminating potential technical artifacts of DNA-methylation array probes.Am J Hum Genet. 2012 Oct 5;91(4):760-2. doi: 10.1016/j.ajhg.2012.05.028. Am J Hum Genet. 2012. PMID: 23040498 Free PMC article. No abstract available.
-
Cross-reactive DNA microarray probes lead to false discovery of autosomal sex-associated DNA methylation.Am J Hum Genet. 2012 Oct 5;91(4):762-4. doi: 10.1016/j.ajhg.2012.06.020. Am J Hum Genet. 2012. PMID: 23040499 Free PMC article. No abstract available.
Similar articles
-
Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex.Nat Neurosci. 2016 Jan;19(1):40-7. doi: 10.1038/nn.4181. Epub 2015 Nov 30. Nat Neurosci. 2016. PMID: 26619358 Free PMC article.
-
Trends in DNA Methylation with Age Replicate Across Diverse Human Populations.Genetics. 2017 Jul;206(3):1659-1674. doi: 10.1534/genetics.116.195594. Epub 2017 May 22. Genetics. 2017. PMID: 28533441 Free PMC article.
-
Distinct DNA methylation changes highly correlated with chronological age in the human brain.Hum Mol Genet. 2011 Mar 15;20(6):1164-72. doi: 10.1093/hmg/ddq561. Epub 2011 Jan 7. Hum Mol Genet. 2011. PMID: 21216877 Free PMC article.
-
Reconfiguration of DNA methylation in aging.Mech Ageing Dev. 2015 Nov;151:60-70. doi: 10.1016/j.mad.2015.02.002. Epub 2015 Feb 20. Mech Ageing Dev. 2015. PMID: 25708826 Review.
-
Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia.Genes (Basel). 2023 Jan 18;14(2):243. doi: 10.3390/genes14020243. Genes (Basel). 2023. PMID: 36833173 Free PMC article. Review.
Cited by
-
The genome-wide DNA methylation profiles among neurosurgery patients with and without post-operative delirium.Psychiatry Clin Neurosci. 2023 Jan;77(1):48-55. doi: 10.1111/pcn.13495. Epub 2022 Nov 17. Psychiatry Clin Neurosci. 2023. PMID: 36266784 Free PMC article.
-
Coordinated Expression of Phosphoinositide Metabolic Genes during Development and Aging of Human Dorsolateral Prefrontal Cortex.PLoS One. 2015 Jul 13;10(7):e0132675. doi: 10.1371/journal.pone.0132675. eCollection 2015. PLoS One. 2015. PMID: 26168237 Free PMC article.
-
Aging effects on DNA methylation modules in human brain and blood tissue.Genome Biol. 2012 Oct 3;13(10):R97. doi: 10.1186/gb-2012-13-10-r97. Genome Biol. 2012. PMID: 23034122 Free PMC article.
-
Epigenetics of Delirium and Aging: Potential Role of DNA Methylation Change on Cytokine Genes in Glia and Blood Along With Aging.Front Aging Neurosci. 2018 Oct 23;10:311. doi: 10.3389/fnagi.2018.00311. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30405391 Free PMC article.
-
FastDMA: an infinium humanmethylation450 beadchip analyzer.PLoS One. 2013 Sep 5;8(9):e74275. doi: 10.1371/journal.pone.0074275. eCollection 2013. PLoS One. 2013. PMID: 24040221 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

