Optimality in the development of intestinal crypts
- PMID: 22304925
- PMCID: PMC3696183
- DOI: 10.1016/j.cell.2011.12.025
Optimality in the development of intestinal crypts
Abstract
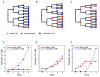
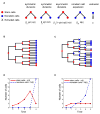
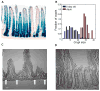
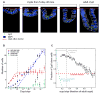
Intestinal crypts in mammals are comprised of long-lived stem cells and shorter-lived progenies. These two populations are maintained in specific proportions during adult life. Here, we investigate the design principles governing the dynamics of these proportions during crypt morphogenesis. Using optimal control theory, we show that a proliferation strategy known as a "bang-bang" control minimizes the time to obtain a mature crypt. This strategy consists of a surge of symmetric stem cell divisions, establishing the entire stem cell pool first, followed by a sharp transition to strictly asymmetric stem cell divisions, producing nonstem cells with a delay. We validate these predictions using lineage tracing and single-molecule fluorescence in situ hybridization of intestinal crypts in infant mice, uncovering small crypts that are entirely composed of Lgr5-labeled stem cells, which become a minority as crypts continue to grow. Our approach can be used to uncover similar design principles in other developmental systems.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells.Cell. 2010 Oct 1;143(1):134-44. doi: 10.1016/j.cell.2010.09.016. Cell. 2010. PMID: 20887898
-
Use of l-pNIPAM hydrogel as a 3D-scaffold for intestinal crypts and stem cell tissue engineering.Biomater Sci. 2019 Sep 24;7(10):4310-4324. doi: 10.1039/c9bm00541b. Biomater Sci. 2019. PMID: 31410428
-
Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation.Nature. 2009 Jan 29;457(7229):603-7. doi: 10.1038/nature07589. Epub 2008 Dec 17. Nature. 2009. PMID: 19092805 Free PMC article.
-
Small intestinal stem cell markers.J Anat. 2008 Jul;213(1):52-8. doi: 10.1111/j.1469-7580.2008.00925.x. J Anat. 2008. PMID: 18638070 Free PMC article. Review.
-
Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis.J Cell Biochem. 2017 May;118(5):943-952. doi: 10.1002/jcb.25719. Epub 2017 Jan 11. J Cell Biochem. 2017. PMID: 27584938 Free PMC article. Review.
Cited by
-
Intestinal stem cell function in Drosophila and mice.Curr Opin Genet Dev. 2012 Aug;22(4):354-60. doi: 10.1016/j.gde.2012.04.002. Epub 2012 May 19. Curr Opin Genet Dev. 2012. PMID: 22608824 Free PMC article. Review.
-
Bending gradients: how the intestinal stem cell gets its home.Cell. 2015 Apr 23;161(3):569-580. doi: 10.1016/j.cell.2015.03.041. Epub 2015 Apr 9. Cell. 2015. PMID: 25865482 Free PMC article.
-
A Model for Adult Organ Resizing Demonstrates Stem Cell Scaling through a Tunable Commitment Rate.Biophys J. 2017 Jul 11;113(1):174-184. doi: 10.1016/j.bpj.2017.05.040. Biophys J. 2017. PMID: 28700915 Free PMC article.
-
Muscle Stem Cells Exhibit Distinct Clonal Dynamics in Response to Tissue Repair and Homeostatic Aging.Cell Stem Cell. 2018 Jan 4;22(1):119-127.e3. doi: 10.1016/j.stem.2017.11.009. Epub 2017 Dec 14. Cell Stem Cell. 2018. PMID: 29249462 Free PMC article.
-
Effects of dose rates on radiation-induced replenishment of intestinal stem cells determined by Lgr5 lineage tracing.J Radiat Res. 2015 Jul;56(4):615-22. doi: 10.1093/jrr/rrv012. Epub 2015 Apr 1. J Radiat Res. 2015. PMID: 25832104 Free PMC article.
References
-
- Al-Nafussi AI, Wright NA. Cell kinetics in the mouse small intestine during immediate postnatal life. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;40:51–62. - PubMed
-
- Alon U. An Introduction to Systems Biology: Design Principles of Biological Circuits. Chapman and Hall/CRC; 2006.
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. II. Evidence from Paneth cells in the newborn mouse. Am J Anat. 1981;160:65–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

