Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia
- PMID: 22303002
- PMCID: PMC3322859
- DOI: 10.1074/jbc.M111.324616
Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia
Abstract
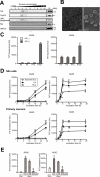
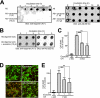
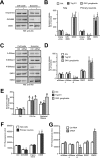
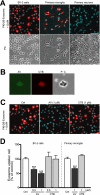
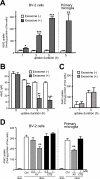
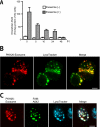
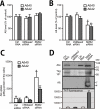
Amyloid β-peptide (Aβ), the pathogenic agent of Alzheimer disease, is a physiological metabolite whose levels are constantly controlled in normal brain. Recent studies have demonstrated that a fraction of extracellular Aβ is associated with exosomes, small membrane vesicles of endosomal origin, although the fate of Aβ in association with exosome is largely unknown. In this study, we identified novel roles for neuron-derived exosomes acting on extracellular Aβ, i.e. exosomes drive conformational changes in Aβ to form nontoxic amyloid fibrils and promote uptake of Aβ by microglia. The Aβ internalized together with exosomes was further transported to lysosomes and degraded. We also found that blockade of phosphatidylserine on the surface of exosomes by annexin V not only prevented exosome uptake but also suppressed Aβ incorporation into microglia. In addition, we demonstrated that secretion of neuron-derived exosomes was modulated by the activities of sphingolipid-metabolizing enzymes, including neutral sphingomyelinase 2 (nSMase2) and sphingomyelin synthase 2 (SMS2). In transwell experiments, up-regulation of exosome secretion from neuronal cells by treatment with SMS2 siRNA enhanced Aβ uptake into microglial cells and significantly decreased extracellular levels of Aβ. Our findings indicate a novel mechanism responsible for clearance of Aβ through its association with exosomes. The modulation of the vesicle release and/or elimination may alter the risk of AD.
Figures
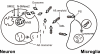
Similar articles
-
Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity.Theranostics. 2021 Feb 25;11(9):4351-4362. doi: 10.7150/thno.52436. eCollection 2021. Theranostics. 2021. PMID: 33754065 Free PMC article.
-
Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice.J Biol Chem. 2014 Aug 29;289(35):24488-98. doi: 10.1074/jbc.M114.577213. Epub 2014 Jul 18. J Biol Chem. 2014. PMID: 25037226 Free PMC article.
-
The Microglial membrane receptor TREM2 mediates exosome secretion to promote phagocytosis of amyloid-β by microglia.FEBS Lett. 2022 Apr;596(8):1059-1071. doi: 10.1002/1873-3468.14336. Epub 2022 Mar 23. FEBS Lett. 2022. PMID: 35292963
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Vesicular Transport of Encapsulated microRNA between Glial and Neuronal Cells.Int J Mol Sci. 2020 Jul 18;21(14):5078. doi: 10.3390/ijms21145078. Int J Mol Sci. 2020. PMID: 32708414 Free PMC article. Review.
Cited by
-
ISGylation is induced in neurons by demyelination driving ISG15-dependent microglial activation.J Neuroinflammation. 2022 Oct 20;19(1):258. doi: 10.1186/s12974-022-02618-4. J Neuroinflammation. 2022. PMID: 36261842 Free PMC article.
-
Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer's Disease.Front Mol Neurosci. 2020 May 29;13:79. doi: 10.3389/fnmol.2020.00079. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32547364 Free PMC article. Review.
-
Extracellular Vesicles in the Development of Cancer Therapeutics.Int J Mol Sci. 2020 Aug 24;21(17):6097. doi: 10.3390/ijms21176097. Int J Mol Sci. 2020. PMID: 32847103 Free PMC article. Review.
-
Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity.Theranostics. 2021 Feb 25;11(9):4351-4362. doi: 10.7150/thno.52436. eCollection 2021. Theranostics. 2021. PMID: 33754065 Free PMC article.
-
Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice.Diabetes. 2016 Oct;65(10):3111-28. doi: 10.2337/db15-1563. Epub 2016 Jun 9. Diabetes. 2016. PMID: 27284111 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

