The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment
- PMID: 22302786
- PMCID: PMC3337709
- DOI: 10.1161/CIRCRESAHA.111.261784
The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment
Abstract
Rationale: Atherosclerosis is a disease of large- and medium-sized arteries that is characterized by chronic vascular inflammation. While the role of Th1, Th2, and T-regulatory subsets in atherogenesis is established, the involvement of IL-17A-producing cells remains unclear.
Objective: To investigate the role of the IL-17A/IL-17RA axis in atherosclerosis.
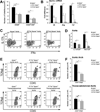
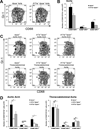
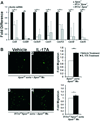
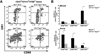
Methods and results: We bred apolipoprotein-E-deficient (Apoe(-/-)) mice with IL-17A-deficient and IL-17 receptor A-deficient mice to generate Il17a(-/-)Apoe(-/-) and Il17ra(-/-)Apoe(-/-) mice. Western diet fed Il17a(-/-)Apoe(-/-) and Il17ra(-/-)Apoe(-/-) mice had smaller atherosclerotic plaques in the aortic arch and aortic roots, but showed little difference in plaque burden in the thoracoabdominal aorta in comparison with Apoe(-/-) controls. Flow cytometric analysis of Il17a(-/-)Apoe(-/-) and Il17ra(-/-)Apoe(-/-) aortas revealed that deficiency of IL-17A/IL-17RA preferentially reduced aortic arch, but not thoracoabdominal aortic T cell, neutrophil, and macrophage content in comparison with Apoe(-/-) aortic segments. In contrast to ubiquitous IL-17RA expression throughout the aorta, IL-17A was preferentially expressed within the aortic arch of WD-fed Apoe(-/-) mice. Deficiency of IL-17A or IL-17RA reduced aortic arch, but not thoracoabdominal aortic TNFα and CXCL2 expression. Aortic vascular IL-17RA supports monocyte adherence to explanted aortas in ex vivo adhesion assays. Short-term homing experiments revealed that the recruitment of adoptively transferred monocytes and neutrophils to the aortas of Il17ra(-/-)Apoe(-/-) mice is impaired in comparison with Apoe(-/-) recipients.
Conclusions: The IL-17A/IL-17RA axis increases aortic arch inflammation during atherogenesis through the induction of aortic chemokines, and the acceleration of neutrophil and monocyte recruitment to this site.
Figures




Similar articles
-
Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A+ T Cells to the Aorta.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1496-506. doi: 10.1161/ATVBAHA.116.307892. Epub 2016 Jun 30. Arterioscler Thromb Vasc Biol. 2016. PMID: 27365405 Free PMC article.
-
Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice.Arterioscler Thromb Vasc Biol. 2012 Feb;32(2):273-80. doi: 10.1161/ATVBAHA.111.229997. Epub 2011 Nov 23. Arterioscler Thromb Vasc Biol. 2012. PMID: 22116098
-
Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice.Circulation. 2010 Apr 20;121(15):1746-55. doi: 10.1161/CIRCULATIONAHA.109.924886. Epub 2010 Apr 5. Circulation. 2010. PMID: 20368519 Free PMC article.
-
Interleukin 17A in atherosclerosis - Regulation and pathophysiologic effector function.Cytokine. 2019 Oct;122:154089. doi: 10.1016/j.cyto.2017.06.016. Epub 2017 Jun 26. Cytokine. 2019. PMID: 28663097 Review.
-
Current views on the functions of interleukin-17A-producing cells in atherosclerosis.Thromb Haemost. 2011 Nov;106(5):787-95. doi: 10.1160/TH11-05-0342. Epub 2011 Sep 22. Thromb Haemost. 2011. PMID: 21946932 Free PMC article. Review.
Cited by
-
Preparation of Single Cell Suspensions from Mouse Aorta.Bio Protoc. 2016 Jun 5;6(11):e1832. doi: 10.21769/bioprotoc.1832. Bio Protoc. 2016. PMID: 27335895 Free PMC article.
-
Preoperative clinical characteristics and risk assessment in Sun's modified classification of Stanford type A acute aortic dissection.BMC Cardiovasc Disord. 2024 Oct 14;24(1):556. doi: 10.1186/s12872-024-04145-x. BMC Cardiovasc Disord. 2024. PMID: 39402485 Free PMC article.
-
Evidence for Biologic Drug Modifying Anti-Rheumatoid Drugs and Association with Cardiovascular Disease Risk Mitigation in Inflammatory Arthritis.Rheum Dis Clin North Am. 2023 Feb;49(1):165-178. doi: 10.1016/j.rdc.2022.08.005. Rheum Dis Clin North Am. 2023. PMID: 36424023 Free PMC article. Review.
-
Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):1969-1985. doi: 10.1161/ATVBAHA.118.311229. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354262 Free PMC article. Review.
-
A Role of IL-17 in Rheumatoid Arthritis Patients Complicated With Atherosclerosis.Front Pharmacol. 2022 Feb 8;13:828933. doi: 10.3389/fphar.2022.828933. eCollection 2022. Front Pharmacol. 2022. PMID: 35211020 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

