Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases
- PMID: 22300978
- PMCID: PMC3281569
- DOI: 10.1101/cshperspect.a011908
Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases
Abstract
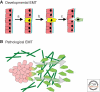
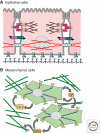
Epithelial-mesenchymal transition (EMT) is a physiological process in which epithelial cells acquire the motile and invasive characteristics of mesenchymal cells. Although EMT in embryonic development is a coordinated, organized process involving interaction between many different cells and tissue types, aspects of the EMT program can be inappropriately activated in response to microenvironmental alterations and aberrant stimuli, and this can contribute to disease conditions including tissue fibrosis and cancer progression. Here we will outline how EMT functions in normal development, how it could be activated in pathologic conditions-especially by matrix metalloproteinases-and how it may be targeted for therapeutic benefit.
Figures


Similar articles
-
Involvement of partial EMT in cancer progression.J Biochem. 2018 Oct 1;164(4):257-264. doi: 10.1093/jb/mvy047. J Biochem. 2018. PMID: 29726955 Review.
-
Epithelial-mesenchymal plasticity: emerging parallels between tissue morphogenesis and cancer metastasis.Philos Trans R Soc Lond B Biol Sci. 2020 Oct 12;375(1809):20200087. doi: 10.1098/rstb.2020.0087. Epub 2020 Aug 24. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32829692 Free PMC article. Review.
-
Epithelial Plasticity During Human Breast Morphogenesis and Cancer Progression.J Mammary Gland Biol Neoplasia. 2016 Dec;21(3-4):139-148. doi: 10.1007/s10911-016-9366-3. Epub 2016 Nov 4. J Mammary Gland Biol Neoplasia. 2016. PMID: 27815674 Free PMC article. Review.
-
[Epithelial mesenchymal transition during development in fibrosis and in the progression of carcinoma].Bull Cancer. 2010 Nov;97(11):1285-95. doi: 10.1684/bdc.2010.1206. Bull Cancer. 2010. PMID: 21084241 Review. French.
-
Basement membrane fragments in the context of the epithelial-to-mesenchymal transition.Eur J Cell Biol. 2016 Nov;95(11):427-440. doi: 10.1016/j.ejcb.2016.06.002. Epub 2016 Jun 18. Eur J Cell Biol. 2016. PMID: 27397693 Review.
Cited by
-
Triggering the landslide: The tumor-promotional effects of myofibroblasts.Exp Cell Res. 2013 Jul 1;319(11):1657-62. doi: 10.1016/j.yexcr.2013.03.015. Epub 2013 Mar 22. Exp Cell Res. 2013. PMID: 23528452 Free PMC article. Review.
-
Analysis of regulator of G-protein signalling 2 (RGS2) expression and function during prostate cancer progression.Sci Rep. 2018 Nov 22;8(1):17259. doi: 10.1038/s41598-018-35332-4. Sci Rep. 2018. PMID: 30467386 Free PMC article.
-
Understanding Multicellularity: The Functional Organization of the Intercellular Space.Front Physiol. 2019 Sep 18;10:1170. doi: 10.3389/fphys.2019.01170. eCollection 2019. Front Physiol. 2019. PMID: 31620013 Free PMC article.
-
Matrix Metalloproteinase 13 from Satellite Cells is Required for Efficient Muscle Growth and Regeneration.Cell Physiol Biochem. 2020 Apr 11;54(3):333-353. doi: 10.33594/000000223. Cell Physiol Biochem. 2020. PMID: 32275813 Free PMC article.
-
Common profiles of Notch signaling differentiate disease-free survival in luminal type A and triple negative breast cancer.Oncotarget. 2017 Jan 24;8(4):6013-6032. doi: 10.18632/oncotarget.13451. Oncotarget. 2017. PMID: 27888801 Free PMC article.
References
-
- Barrallo-Gimeno A, Nieto MA 2005. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 132: 3151–3161 - PubMed
-
- Bartow SA, Pathak DR, Mettler FA 1990. Radiographic microcalcification and parenchymal patterns as indicators of histologic “high-risk” benign breast disease. Cancer 66: 1721–1725 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
