HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication
- PMID: 22291597
- PMCID: PMC3266935
- DOI: 10.1371/journal.ppat.1002502
HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication
Abstract
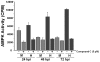
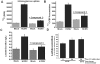
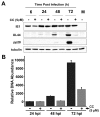
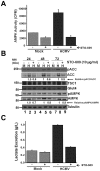
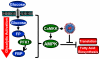
Human Cytomegalovirus (HCMV) infection induces several metabolic activities that have been found to be important for viral replication. The cellular AMP-activated protein kinase (AMPK) is a metabolic stress response kinase that regulates both energy-producing catabolic processes and energy-consuming anabolic processes. Here we explore the role AMPK plays in generating an environment conducive to HCMV replication. We find that HCMV infection induces AMPK activity, resulting in the phosphorylation and increased abundance of several targets downstream of activated AMPK. Pharmacological and RNA-based inhibition of AMPK blocked the glycolytic activation induced by HCMV-infection, but had little impact on the glycolytic pathway of uninfected cells. Furthermore, inhibition of AMPK severely attenuated HCMV replication suggesting that AMPK is an important cellular factor for HCMV replication. Inhibition of AMPK attenuated early and late gene expression as well as viral DNA synthesis, but had no detectable impact on immediate-early gene expression, suggesting that AMPK activity is important at the immediate early to early transition of viral gene expression. Lastly, we find that inhibition of the Ca²⁺-calmodulin-dependent kinase kinase (CaMKK), a kinase known to activate AMPK, blocks HCMV-mediated AMPK activation. The combined data suggest a model in which HCMV activates AMPK through CaMKK, and depends on their activation for high titer replication, likely through induction of a metabolic environment conducive to viral replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
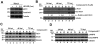


Similar articles
-
Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny.J Virol. 2011 Jan;85(2):705-14. doi: 10.1128/JVI.01557-10. Epub 2010 Nov 17. J Virol. 2011. PMID: 21084482 Free PMC article.
-
Digitoxin Suppresses Human Cytomegalovirus Replication via Na+, K+/ATPase α1 Subunit-Dependent AMP-Activated Protein Kinase and Autophagy Activation.J Virol. 2018 Feb 26;92(6):e01861-17. doi: 10.1128/JVI.01861-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29321306 Free PMC article.
-
Human Cytomegalovirus Induces the Expression of the AMPKa2 Subunit to Drive Glycolytic Activation and Support Productive Viral Infection.J Virol. 2021 Mar 1;95(5):e01321-20. doi: 10.1128/JVI.01321-20. Epub 2020 Dec 2. J Virol. 2021. PMID: 33268515 Free PMC article.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
-
Drug targets in cytomegalovirus infection.Infect Disord Drug Targets. 2009 Apr;9(2):201-22. doi: 10.2174/187152609787847758. Infect Disord Drug Targets. 2009. PMID: 19275707 Review.
Cited by
-
Potential of protein kinase inhibitors for treating herpesvirus-associated disease.Trends Microbiol. 2013 Jun;21(6):286-95. doi: 10.1016/j.tim.2013.03.005. Epub 2013 Apr 19. Trends Microbiol. 2013. PMID: 23608036 Free PMC article. Review.
-
IFI16 Impacts Metabolic Reprogramming during Human Cytomegalovirus Infection.mBio. 2022 Jun 28;13(3):e0043522. doi: 10.1128/mbio.00435-22. Epub 2022 Apr 14. mBio. 2022. PMID: 35420480 Free PMC article.
-
Fatty Acids Regulate Porcine Reproductive and Respiratory Syndrome Virus Infection via the AMPK-ACC1 Signaling Pathway.Viruses. 2019 Dec 10;11(12):1145. doi: 10.3390/v11121145. Viruses. 2019. PMID: 31835577 Free PMC article.
-
AMP-Activated Protein Kinase and Host Defense against Infection.Int J Mol Sci. 2018 Nov 6;19(11):3495. doi: 10.3390/ijms19113495. Int J Mol Sci. 2018. PMID: 30404221 Free PMC article. Review.
-
Differential Metabolic Reprogramming by Zika Virus Promotes Cell Death in Human versus Mosquito Cells.Cell Metab. 2019 May 7;29(5):1206-1216.e4. doi: 10.1016/j.cmet.2019.01.024. Epub 2019 Feb 28. Cell Metab. 2019. PMID: 30827860 Free PMC article.
References
-
- Litman RM, Pardee AB. Production of bacteriophage mutants by a disturbance of deoxyribonucleic acid metabolism. Nature. 1956;178:529–531. - PubMed
-
- Pearson HE, Winzler RJ. Oxidative and glycolytic metabolism of minced day-old mouse brain in relation to propagation of Theiler's GD VII virus. J Biol Chem. 1949;181:577–582. - PubMed
-
- Salzman NP, Lockart RZ, Jr, Sebring ED. Alterations in HeLa cell metabolism resulting from poliovirus infection. Virology. 1959;9:244–259. - PubMed
-
- Kaplan AS, Ben-Porat T. The action of 5-fluorouracil on the nucleic acid metabolism of pseudorabies virus-infected and noninfected rabbit kidney cells. Virology. 1961;13:78–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

