Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice
- PMID: 22291136
- PMCID: PMC6390958
- DOI: 10.1158/1078-0432.CCR-11-3050
Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice
Abstract
Purpose: We investigated the feasibility of delivering the proinflammatory cytokine interleukin (IL)-12 into tumor using T cells genetically engineered to express a chimeric antigen receptor (CAR) against the VEGF receptor-2 (VEGFR-2).
Experimental design: Two different strains of mice bearing five different established subcutaneous tumors were treated with syngeneic T cells cotransduced with an anti-VEGFR-2 CAR and a constitutively expressed single-chain murine IL-12 or an inducible IL-12 gene after host lymphodepletion. Tumor regression, survival of mice, and persistence of the transferred cells were evaluated.
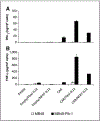
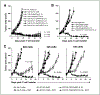
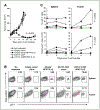
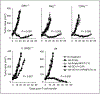
Results: Adoptive transfer of syngeneic T cells cotransduced with an anti-VEGFR-2 CAR and a constitutively expressing single-chain IL-12 resulted in the regression of five different established tumors of different histologies without the need for IL-2 administration. T cells transduced with either anti-VEGFR-2 CAR or single-chain IL-12 alone did not alter the tumor growth indicating that both of them had to be expressed in the same cell to mediate tumor regression. Anti-VEGFR-2 CAR and IL-12-cotransduced T cells infiltrated the tumors, expanded, and persisted for prolonged periods. The antitumor effect did not require the presence of host T and B cells but was dependent on host IL-12R-expressing cells. The anti-VEGFR-2 CAR changed the immunosuppressive tumor environment by altering/reducing both the systemic and the intratumoral CD11b(+)Gr1(+) myeloid suppressor cell subsets that expressed VEGFR-2.
Conclusions: These results suggest that targeted delivery of IL-12 into the tumor environment with T cells redirected against VEGFR-2 is a promising approach for treating patients with a variety of solid tumor types.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice.J Clin Invest. 2010 Nov;120(11):3953-68. doi: 10.1172/JCI43490. Epub 2010 Oct 11. J Clin Invest. 2010. PMID: 20978347 Free PMC article.
-
Enhanced efficacy and limited systemic cytokine exposure with membrane-anchored interleukin-12 T-cell therapy in murine tumor models.J Immunother Cancer. 2020 Jan;8(1):e000210. doi: 10.1136/jitc-2019-000210. J Immunother Cancer. 2020. PMID: 31959727 Free PMC article.
-
Treatment of experimental breast cancer using interleukin-12 gene therapy combined with anti-vascular endothelial growth factor receptor-2 antibody.Mol Cancer Ther. 2004 Aug;3(8):969-76. Mol Cancer Ther. 2004. PMID: 15299079
-
Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma.Immunol Rev. 2014 Jan;257(1):83-90. doi: 10.1111/imr.12125. Immunol Rev. 2014. PMID: 24329791 Review.
-
Chimeric antigen receptor T cells: a novel therapy for solid tumors.J Hematol Oncol. 2017 Mar 29;10(1):78. doi: 10.1186/s13045-017-0444-9. J Hematol Oncol. 2017. PMID: 28356156 Free PMC article. Review.
Cited by
-
Discovery of immunotherapy targets for pediatric solid and brain tumors by exon-level expression.Res Sq [Preprint]. 2024 Jan 5:rs.3.rs-3821632. doi: 10.21203/rs.3.rs-3821632/v1. Res Sq. 2024. Update in: Nat Commun. 2024 May 3;15(1):3732. doi: 10.1038/s41467-024-47649-y. PMID: 38260279 Free PMC article. Updated. Preprint.
-
Targeting the tumor vasculature to enhance T cell activity.Curr Opin Immunol. 2015 Apr;33:55-63. doi: 10.1016/j.coi.2015.01.011. Epub 2015 Feb 6. Curr Opin Immunol. 2015. PMID: 25665467 Free PMC article. Review.
-
The Great War of Today: Modifications of CAR-T Cells to Effectively Combat Malignancies.Cancers (Basel). 2020 Jul 24;12(8):2030. doi: 10.3390/cancers12082030. Cancers (Basel). 2020. PMID: 32722109 Free PMC article. Review.
-
IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models.J Clin Invest. 2023 May 1;133(9):e166028. doi: 10.1172/JCI166028. J Clin Invest. 2023. PMID: 36951942 Free PMC article.
-
Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells.Biomater Sci. 2019 Mar 26;7(4):1345-1357. doi: 10.1039/c8bm01556b. Biomater Sci. 2019. PMID: 30698174 Free PMC article.
References
-
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999;5:1359–64. - PubMed
-
- Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D, et al. Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6. Clin Cancer Res 1996;2:641–52. - PubMed
-
- Ganss R, Limmer A, Sacher T, Arnold B, Hammerling GJ. Autoaggression and tumor rejection: it takes more than self-specific T-cell activation. Immunol Rev 1999;169:263–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

