The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2
- PMID: 22291037
- PMCID: PMC3275380
- DOI: 10.1083/jcb.201107115
The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2
Abstract
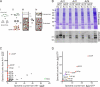
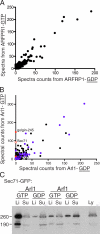
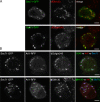
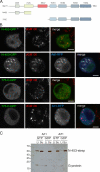
The small G protein Arf1 regulates Golgi traffic and is activated by two related types of guanine nucleotide exchange factor (GEF). GBF1 acts at the cis-Golgi, whereas BIG1 and its close paralog BIG2 act at the trans-Golgi. Peripheral membrane proteins such as these GEFs are often recruited to membranes by small G proteins, but the basis for specific recruitment of Arf GEFs, and hence Arfs, to Golgi membranes is not understood. In this paper, we report a liposome-based affinity purification method to identify effectors for small G proteins of the Arf family. We validate this with the Drosophila melanogaster Arf1 orthologue (Arf79F) and the related class II Arf (Arf102F), which showed a similar pattern of effector binding. Applying the method to the Arf-like G protein Arl1, we found that it binds directly to Sec71, the Drosophila ortholog of BIG1 and BIG2, via an N-terminal region. We show that in mammalian cells, Arl1 is necessary for Golgi recruitment of BIG1 and BIG2 but not GBF1. Thus, Arl1 acts to direct a trans-Golgi-specific Arf1 GEF, and hence active Arf1, to the trans side of the Golgi.
© 2012 Christis and Munro
Figures
Similar articles
-
Structural Insights into Arl1-Mediated Targeting of the Arf-GEF BIG1 to the trans-Golgi.Cell Rep. 2016 Jul 19;16(3):839-50. doi: 10.1016/j.celrep.2016.06.022. Epub 2016 Jun 30. Cell Rep. 2016. PMID: 27373159 Free PMC article.
-
The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN).J Biol Chem. 2013 Apr 19;288(16):11532-45. doi: 10.1074/jbc.M112.438481. Epub 2013 Feb 5. J Biol Chem. 2013. PMID: 23386609 Free PMC article.
-
Promiscuity of the catalytic Sec7 domain within the guanine nucleotide exchange factor GBF1 in ARF activation, Golgi homeostasis, and effector recruitment.Mol Biol Cell. 2019 Jun 1;30(12):1523-1535. doi: 10.1091/mbc.E18-11-0711. Epub 2019 Apr 3. Mol Biol Cell. 2019. PMID: 30943106 Free PMC article.
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
-
Allosteric regulation of Arf GTPases and their GEFs at the membrane interface.Small GTPases. 2016 Oct;7(4):283-296. doi: 10.1080/21541248.2016.1215778. Epub 2016 Jul 22. Small GTPases. 2016. PMID: 27449855 Free PMC article. Review.
Cited by
-
ARF GTPases and their GEFs and GAPs: concepts and challenges.Mol Biol Cell. 2019 May 15;30(11):1249-1271. doi: 10.1091/mbc.E18-12-0820. Mol Biol Cell. 2019. PMID: 31084567 Free PMC article. Review.
-
GARP dysfunction results in COPI displacement, depletion of Golgi v-SNAREs and calcium homeostasis proteins.Front Cell Dev Biol. 2022 Dec 12;10:1066504. doi: 10.3389/fcell.2022.1066504. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578782 Free PMC article.
-
Kinetics of Arf1 inactivation regulates Golgi organisation and function in non-adherent fibroblasts.Biol Open. 2023 Apr 15;12(4):bio059669. doi: 10.1242/bio.059669. Epub 2023 May 4. Biol Open. 2023. PMID: 36946871 Free PMC article.
-
The Vac14-interaction network is linked to regulators of the endolysosomal and autophagic pathway.Mol Cell Proteomics. 2014 Jun;13(6):1397-411. doi: 10.1074/mcp.M113.034108. Epub 2014 Feb 27. Mol Cell Proteomics. 2014. PMID: 24578385 Free PMC article.
-
The HUS box is required for allosteric regulation of the Sec7 Arf-GEF.J Biol Chem. 2018 May 4;293(18):6682-6691. doi: 10.1074/jbc.RA117.001318. Epub 2018 Mar 7. J Biol Chem. 2018. PMID: 29514977 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

