Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons
- PMID: 22291024
- PMCID: PMC3308742
- DOI: 10.1074/jbc.M111.314385
Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons
Abstract
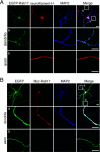
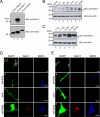
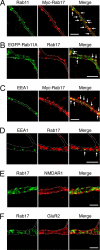
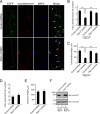
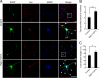
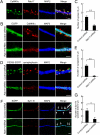
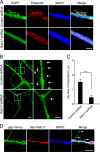
Neurons are compartmentalized into two morphologically, molecularly, and functionally distinct domains: axons and dendrites, and precise targeting and localization of proteins within these domains are critical for proper neuronal functions. It has been reported that several members of the Rab family small GTPases that are key mediators of membrane trafficking, regulate axon-specific trafficking events, but little has been elucidated regarding the molecular mechanisms that underlie dendrite-specific membrane trafficking. Here we show that Rab17 regulates dendritic morphogenesis and postsynaptic development in mouse hippocampal neurons. Rab17 is localized at dendritic growth cones, shafts, filopodia, and mature spines, but it is mostly absent in axons. We also found that Rab17 mediates dendrite growth and branching and that it does not regulate axon growth or branching. Moreover, shRNA-mediated knockdown of Rab17 expression resulted in a dramatically reduced number of dendritic spines, probably because of impaired filopodia formation. These findings have revealed the first molecular link between membrane trafficking and dendritogenesis.
Figures
Similar articles
-
Critical importance of RAB proteins for synaptic function.Small GTPases. 2018 Mar 4;9(1-2):145-157. doi: 10.1080/21541248.2016.1277001. Epub 2017 Apr 13. Small GTPases. 2018. PMID: 28146371 Free PMC article. Review.
-
Assay of Rab17 and its guanine nucleotide exchange factor Rabex-5 in the dendrites of hippocampal neurons.Methods Mol Biol. 2015;1298:233-43. doi: 10.1007/978-1-4939-2569-8_20. Methods Mol Biol. 2015. PMID: 25800847
-
Small GTPase Rab17 regulates the surface expression of kainate receptors but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in hippocampal neurons via dendritic trafficking of Syntaxin-4 protein.J Biol Chem. 2014 Jul 25;289(30):20773-87. doi: 10.1074/jbc.M114.550632. J Biol Chem. 2014. PMID: 24895134 Free PMC article.
-
Rabex-5 protein regulates dendritic localization of small GTPase Rab17 and neurite morphogenesis in hippocampal neurons.J Biol Chem. 2013 Apr 5;288(14):9835-9847. doi: 10.1074/jbc.M112.427591. Epub 2013 Feb 19. J Biol Chem. 2013. PMID: 23430262 Free PMC article.
-
Rab family small GTPases-mediated regulation of intracellular logistics in neural development.Histol Histopathol. 2018 Aug;33(8):765-771. doi: 10.14670/HH-11-956. Epub 2017 Dec 19. Histol Histopathol. 2018. PMID: 29266163 Review.
Cited by
-
Invasive cells in animals and plants: searching for LECA machineries in later eukaryotic life.Biol Direct. 2013 Apr 4;8:8. doi: 10.1186/1745-6150-8-8. Biol Direct. 2013. PMID: 23557484 Free PMC article. Review.
-
Rab GTPases in the differential processing of phagocytosed pathogens versus efferocytosed apoptotic cells.Histol Histopathol. 2021 Feb;36(2):123-135. doi: 10.14670/HH-18-252. Epub 2020 Sep 29. Histol Histopathol. 2021. PMID: 32990320 Review.
-
Single-cell RNA-seq and bulk-seq identify RAB17 as a potential regulator of angiogenesis by human dermal microvascular endothelial cells in diabetic foot ulcers.Burns Trauma. 2023 Aug 18;11:tkad020. doi: 10.1093/burnst/tkad020. eCollection 2023. Burns Trauma. 2023. PMID: 37605780 Free PMC article.
-
The GTPase-deficient Rab27A(Q78L) mutant inhibits melanosome transport in melanocytes through trapping of Rab27A effector protein Slac2-a/melanophilin in their cytosol: development of a novel melanosome-targetinG tag.J Biol Chem. 2014 Apr 18;289(16):11059-11067. doi: 10.1074/jbc.M114.552281. Epub 2014 Feb 28. J Biol Chem. 2014. PMID: 24584932 Free PMC article.
-
Critical importance of RAB proteins for synaptic function.Small GTPases. 2018 Mar 4;9(1-2):145-157. doi: 10.1080/21541248.2016.1277001. Epub 2017 Apr 13. Small GTPases. 2018. PMID: 28146371 Free PMC article. Review.
References
-
- Urbanska M., Blazejczyk M., Jaworski J. (2008) Molecular basis of dendritic arborization. Acta Neurobiol. Exp. 68, 264–288 - PubMed
-
- Yoshihara Y., De Roo M., Muller D. (2009) Dendritic spine formation and stabilization. Curr. Opin. Neurobiol. 19, 146–153 - PubMed
-
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. (1990) Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263 - PubMed
-
- Setou M., Nakagawa T., Seog D. H., Hirokawa N. (2000) Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

