Identification of a redox-sensitive switch within the JAK2 catalytic domain
- PMID: 22281400
- PMCID: PMC3319112
- DOI: 10.1016/j.freeradbiomed.2011.12.025
Identification of a redox-sensitive switch within the JAK2 catalytic domain
Abstract
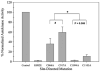
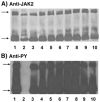
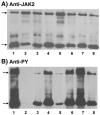
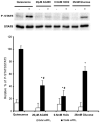
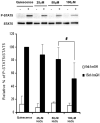
Four cysteine residues (Cys866, Cys917, Cys1094, and Cys1105) have direct roles in cooperatively regulating Janus kinase 2 (JAK2) catalytic activity. Additional site-directed mutagenesis experiments now provide evidence that two of these residues (Cys866 and Cys917) act together as a redox-sensitive switch, allowing JAK2's catalytic activity to be directly regulated by the redox state of the cell. We created several variants of the truncated JAK2 (GST/(NΔ661)rJAK2), which incorporated cysteine-to-serine or cysteine-to-alanine mutations. The catalytic activities of these mutant enzymes were evaluated by in vitro autokinase assays and by in situ autophosphorylation and transphosphorylation assays. Cysteine-to-alanine mutagenesis revealed that the mechanistic role of Cys866 and Cys917 is functionally distinct from that of Cys1094 and Cys1105. Most notable is the observation that the robust activity of the CC866,917AA mutant is unaltered by pretreatment with dithiothreitol or o-iodosobenzoate, unlike all other JAK2 variants previously examined. This work provides the first direct evidence for a cysteine-based redox-sensitive switch that regulates JAK2 catalytic activity. The presence of this redox-sensitive switch predicts that reactive oxygen species can impair the cell's response to JAK-coupled cytokines under conditions of oxidative stress, which we confirm in a murine pancreatic β-islet cell line.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



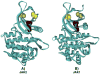
Similar articles
-
Multiple cysteine residues are implicated in Janus kinase 2-mediated catalysis.Biochemistry. 2007 Dec 25;46(51):14810-8. doi: 10.1021/bi701118u. Epub 2007 Dec 4. Biochemistry. 2007. PMID: 18052197
-
Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation.J Biol Chem. 2007 Oct 19;282(42):30707-17. doi: 10.1074/jbc.M702607200. Epub 2007 Aug 29. J Biol Chem. 2007. PMID: 17728251
-
Three Tyrosine Residues in the Erythropoietin Receptor Are Essential for Janus Kinase 2 V617F Mutant-induced Tumorigenesis.J Biol Chem. 2017 Feb 3;292(5):1826-1846. doi: 10.1074/jbc.M116.749465. Epub 2016 Dec 20. J Biol Chem. 2017. PMID: 27998978 Free PMC article.
-
Oxidative stress: the vulnerable beta-cell.Biochem Soc Trans. 2008 Jun;36(Pt 3):343-7. doi: 10.1042/BST0360343. Biochem Soc Trans. 2008. PMID: 18481954 Review.
-
Redox regulation of Janus kinase: The elephant in the room.JAKSTAT. 2013 Oct 1;2(4):e26141. doi: 10.4161/jkst.26141. Epub 2013 Aug 19. JAKSTAT. 2013. PMID: 24416654 Free PMC article. Review.
Cited by
-
Redox regulation of tyrosine kinase signalling: more than meets the eye.J Biochem. 2020 Feb 1;167(2):151-163. doi: 10.1093/jb/mvz085. J Biochem. 2020. PMID: 31599960 Free PMC article. Review.
-
Differential STAT3 signaling in the heart: Impact of concurrent signals and oxidative stress.JAKSTAT. 2012 Apr 1;1(2):101-10. doi: 10.4161/jkst.19776. JAKSTAT. 2012. PMID: 23904970 Free PMC article.
-
Nitric Oxide-Releasing Drug Glyceryl Trinitrate Targets JAK2/STAT3 Signaling, Migration and Invasion of Triple-Negative Breast Cancer Cells.Int J Mol Sci. 2021 Aug 6;22(16):8449. doi: 10.3390/ijms22168449. Int J Mol Sci. 2021. PMID: 34445170 Free PMC article.
-
Redox regulation of cysteine-dependent enzymes in neurodegeneration.Int J Cell Biol. 2012;2012:703164. doi: 10.1155/2012/703164. Epub 2012 Jul 5. Int J Cell Biol. 2012. PMID: 22829832 Free PMC article.
-
Organic dust induces inflammatory gene expression in lung epithelial cells via ROS-dependent STAT-3 activation.Am J Physiol Lung Cell Mol Physiol. 2019 Jul 1;317(1):L127-L140. doi: 10.1152/ajplung.00448.2018. Epub 2019 May 1. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31042082 Free PMC article.
References
-
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. - PubMed
-
- Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. - PubMed
-
- Freedman JE. Oxidative stress and platelets. Arterioscler Thromb Vase Biol. 2008;28:s11–16. - PubMed
-
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

