Analysis of the key elements of FFAT-like motifs identifies new proteins that potentially bind VAP on the ER, including two AKAPs and FAPP2
- PMID: 22276202
- PMCID: PMC3261905
- DOI: 10.1371/journal.pone.0030455
Analysis of the key elements of FFAT-like motifs identifies new proteins that potentially bind VAP on the ER, including two AKAPs and FAPP2
Abstract
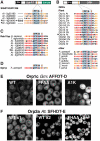
Background: Two phenylalanines (FF) in an acidic tract (FFAT)-motifs were originally described as having seven elements: an acidic flanking region followed by 6 residues (EFFDA-E). Such motifs are found in several lipid transfer protein (LTP) families, and they interact with a protein on the cytosolic face of the ER called vesicle-associated membrane protein-associated protein (VAP). Mutation of which causes ER stress and motor neuron disease, making it important to determine which proteins bind VAP. Among other proteins that bind VAP, some contain FFAT-like motifs that are missing one or more of the seven elements. Defining how much variation is tolerated in FFAT-like motifs is a preliminary step prior to the identification of the full range of VAP interactors.
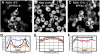
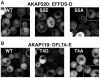
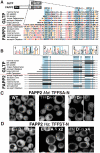
Results: We used a quantifiable in vivo system that measured ER targeting in a reporter yeast strain that over-expressed VAP to study the effect of substituting different elements of FFAT-like motifs in turn. By defining FFAT-like motifs more widely than before, we found them in novel proteins the functions of which had not previously been directly linked to the ER, including: two PKA anchoring proteins, AKAP220 and AKAP110; a family of plant LTPs; and the glycolipid LTP phosphatidylinositol-four-phosphate adaptor-protein-2 (FAPP-2).
Conclusion: All of the seven essential elements of a FFAT motif tolerate variation, and weak targeting to the ER via VAP is still detected if two elements are substituted. In addition to the strong FFAT motifs already known, there are additional proteins with weaker FFAT-like motifs, which might be functionally important VAP interactors.
Conflict of interest statement
Figures
Similar articles
-
A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins.J Biol Chem. 2005 Apr 8;280(14):14097-104. doi: 10.1074/jbc.M500147200. Epub 2005 Jan 24. J Biol Chem. 2005. PMID: 15668246
-
VAP, a Versatile Access Point for the Endoplasmic Reticulum: Review and analysis of FFAT-like motifs in the VAPome.Biochim Biophys Acta. 2016 Aug;1861(8 Pt B):952-961. doi: 10.1016/j.bbalip.2016.02.009. Epub 2016 Feb 17. Biochim Biophys Acta. 2016. PMID: 26898182 Review.
-
Structural basis of FFAT motif-mediated ER targeting.Structure. 2005 Jul;13(7):1035-45. doi: 10.1016/j.str.2005.04.010. Structure. 2005. PMID: 16004875
-
VAP-B binds to Rab3GAP1 at the ER: its implication in nuclear envelope formation through the ER-Golgi intermediate compartment.Kobe J Med Sci. 2014 Oct 1;60(3):E48-56. Kobe J Med Sci. 2014. PMID: 25612670
-
The Interactome of the VAP Family of Proteins: An Overview.Cells. 2021 Jul 14;10(7):1780. doi: 10.3390/cells10071780. Cells. 2021. PMID: 34359948 Free PMC article. Review.
Cited by
-
SEIPIN Isoforms Interact with the Membrane-Tethering Protein VAP27-1 for Lipid Droplet Formation.Plant Cell. 2020 Sep;32(9):2932-2950. doi: 10.1105/tpc.19.00771. Epub 2020 Jul 20. Plant Cell. 2020. PMID: 32690719 Free PMC article.
-
Sphingolipid transfer proteins defined by the GLTP-fold.Q Rev Biophys. 2015 Aug;48(3):281-322. doi: 10.1017/S003358351400016X. Epub 2015 Mar 23. Q Rev Biophys. 2015. PMID: 25797198 Free PMC article.
-
MOSPD2 is an endoplasmic reticulum-lipid droplet tether functioning in LD homeostasis.J Cell Biol. 2022 Jun 6;221(6):e202110044. doi: 10.1083/jcb.202110044. Epub 2022 Apr 7. J Cell Biol. 2022. PMID: 35389430 Free PMC article.
-
Noroviruses Co-opt the Function of Host Proteins VAPA and VAPB for Replication via a Phenylalanine-Phenylalanine-Acidic-Tract-Motif Mimic in Nonstructural Viral Protein NS1/2.mBio. 2017 Jul 11;8(4):e00668-17. doi: 10.1128/mBio.00668-17. mBio. 2017. PMID: 28698274 Free PMC article.
-
What the VAP: The Expanded VAP Family of Proteins Interacting With FFAT and FFAT-Related Motifs for Interorganellar Contact.Contact (Thousand Oaks). 2021 May 9;4:25152564211012246. doi: 10.1177/25152564211012246. eCollection 2021 Jan 1. Contact (Thousand Oaks). 2021. PMID: 34036242 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

